More Information
Submitted: 10 July 2019 | Approved: 15 July 2019 | Published: 16 July 2019
How to cite this article: Altenburg J, Wilms EB, Boersma WG. The relationship between serum and sputum levels of azithromycin and clinical endpoints in patients with bronchiectasis using azithromycin maintenance treatment. Arch Pharm Pharma Sci. 2019; 3: 019-025. doi: 10.29328/journal.apps.1001014
Copyright License: © 2019 Altenburg J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The relationship between serum and sputum levels of azithromycin and clinical endpoints in patients with bronchiectasis using azithromycin maintenance treatment
Josje Altenburg1*, Erik B Wilms2 and Wim G Boersma3
1Department of Pulmonary Diseases, Amsterdam University Medical Centres, Amsterdam, the Netherlands
2The Hague Hospital Pharmacy, The Hague, the Netherlands
3Department of Pulmonary Diseases, North West Hospital Group, Alkmaar, the Netherlands
*Address for Correspondence: Dr. J Altenburg, Department of Pulmonary Diseases, Amsterdam University Medical Centres, Amsterdam, The Netherlands, Tel: 0031-20-5664356; Fax: 0031-20-5669001; Email: [email protected]
Background: Azithromycin (AZM) is a macrolide antibiotic with distinct pharmacokinetic properties and is increasingly used as maintenance treatment in patients with bronchiectasis in order to reduce infectious exacerbations and improve pulmonary symptoms. The exact mechanism of action is not known and the relation between azithromycin dose level, local and systemic drug levels and clinical effect however, has not been extensively studied yet.
Objectives: To explore the relation between AZM serum and sputum concentrations, clinical effect parameters and side effects.
Methods: Azithromycin concentrations were measured in serum and sputum samples of bronchiectasis patients receiving one year of AZM treatment (250mg OD) enrolled in the Bronchiectasis and Azithromycin Treatment (BAT) trial, a double blind, randomised placebo-controlled trial. Results were correlated with data on AZM dose level, exacerbation frequency, lung function (forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC), quality of life and symptoms collected within the same year.
Results: 83 sputum samples from 31 patients and 151 serum samples from 43 patients were available for analysis. Mean AZM dose-level ranged from 18.8 to 39.8 mg/kg body weight/ week, generating mean AZM concentrations of 7.57 mg/L (SD 9.49) in sputum and 0.11 mg/L (SD 0.085) in serum. No correlation was found between side effects and AZM dose level, sputum- or serum concentrations. Significant correlation was found between AZM sputum concentration and CRP-level (r= -0.6).
Conclusion: High and stable AZM sputum levels were reached during long term treatment, as opposed to low AZM levels in serum. Apart from CRP-levels to AZM sputum concentration, no other outcome parameter showed significant correlation to AZM serum- or sputum levels. AZM dose- or exposure levels were not predictive for the occurrence of side effects.
Azithromycin (AZM) is a 15-membered azalide antibiotic exhibiting a bacteriostatic effect towards susceptible pathogens (mainly gram positive organisms) through inhibition of the RNA-dependent protein synthesis, attenuation of the bacterial biofilm and a deleterious effect on bacterial virulence factors. In addition, an anti-inflammatory effect is described, involving reduced cytokine production and anti-neutrophilic action among other effects, which remains incompletely understood [1].
AZM has distinct pharmacokinetic properties which set it apart from other macrolide antibiotics. Its large volume of distribution due to rapid uptake and accumulation in phagocytic cells is followed by slow release and accounts for an exceptionally long half-life and high intracellular concentrations in the presence of low plasma levels [1,2].
Short courses (3 to 5 days) of AZM are frequently used to treat a variety of community acquired infections. Long term azithromycin treatment is a key element of Cystic Fibrosis (CF) treatment and is increasingly used to treat other chronic respiratory infections, such as non-CF bronchiectasis (hereafter referred to as ‘bronchiectasis’) and COPD after favourable results of clinical trials [3-6].
Bronchiectasis –abnormal dilated bronchi, resulting from a vicious circle of mucus retention, bacterial colonization and inflammation- is a chronic lung disease, characterized by a variable course. Stable periods with a mild productive cough are interspersed with infectious exacerbations which importantly contribute to reduced quality of life.
Since 2012 three randomised clinical trials have confirmed the efficacy of long term macrolide treatment in bronchiectasis.7-9 Patients treated with azithromycin (250 OD or 500 mg three times weekly) or erythromycin (400 mg BD) showed a marked reduction of infectious exacerbations annually. Favourable effects were also noted with respect to lung function and quality of life but these were not consistent between studies.
The pharmacokinetics (PK) and exposure after a single dose or short courses of azithromycin are well known. Exposure after chronic use in CF patients has been investigated by measuring azithromycin in blood and in sputum. CF patients on chronic azithromycin show a wide inter-individual variation in clinical efficacy but also in blood-, sputum and tissue concentrations of AZM, even at the same dose level. The intra individual variation in sputum concentration showed a stable concentration when measured at monthly intervals during 3 months. A relationship between exposure in blood and sputum and clinical efficacy has never been investigated neither in CF treatment nor in treatment of bronchiectasis. We report the results of a study towards the relationship between individual exposure and clinical efficacy of chronic azithromycin therapy in patients with bronchiectasis.
In the current study the authors explore the relation between AZM concentrations both in plasma and sputum and clinical effect parameters: exacerbation frequency, lung function (Forced Expiratory Volume in 1 second (FEV1) and Forced Vital Capacity (FVC), High Resolution Computed Tomography (HRCT) scores, quality of life and symptoms. Additionally we investigated the relationship between azithromycin dose level in mg/kg bodyweight and exposure, clinical efficacy parameters and side effects.
The ‘BAT’ (Bronchiectasis and Azithromycin Treatment) trial, a multicentre, 1:1 randomised, placebo-controlled trial was conducted at 14 sites in the Netherlands from 2008- 2010 (Clinicaltrials.gov, registration no: NCT00415350). Detailed study protocols are provided elsewhere.7 Participants were eligible for randomization if they had bronchiectasis and three or more lower respiratory tract infections treated with antibiotics in the preceding year, with positive sputum cultures.
All participants gave informed consent and ethical approval was provided by the Institutional review board of Alkmaar Medical Centre: ‘METC Noord Holland’ (Approval no: M07-002, CCMO: NL16025.094.07).
Patients were randomised to receive either azithromycin (250 mg once daily) or placebo for 12 months, during which the number of infectious exacerbations (the primary endpoint), lung function parameters, sputum bacteriology, HRCT-scores, inflammatory markers, adverse effects, symptom scores and quality of life (QOL) were recorded. During the BAT trial an infectious exacerbation was defined as the prescription of a course of antibiotics because of the presence of at least 4 of the following 9 symptoms, signs, or findings: (1) change in sputum production (consistency, colour, volume, or haemoptysis); (2) increased dyspnoea (chest congestion or shortness of breath); (3) increased cough; (4) fever (>38°C); (5) increased wheezing; (6) decreased exercise tolerance, malaise, fatigue, or lethargy; (7) FEV1 or FVC decreased by at least 10% from a previously recorded value; (8) radiographic changes indicative of a new pulmonary infectious process; or (9) changes in chest sounds [7].
All patients were familiar with routine spirometry measurements and these were performed according to European Respiratory Society standard criteria.11 Reference values for spirometry were from the European Coal and Steel Community [8].
Symptoms were measured using visual analogue scales (VAS) for dyspnoea, cough, fatigue, chest pain and sputum purulence. Each symptom was scored from 1 to 10, higher scores indicating more severe symptoms and domain- and total scores were provided [9].
Saint George’s Respiratory Questionnaire (SGRQ) was used to measure health related QoL (HRQoL). Its 76 items are partitioned into three sections (Symptoms, Activity, Impact), yielding domain- and total scores, ranging from 0 to 100%, zero indicating no impairment of quality of life. A difference of 4 points or more is considered clinically significant [10-12].
At baseline and after one year of study treatment, HRCT scans were obtained and independently scored by two radiologists according to the validated scoring system designed by Bhalla et al. [13]. Scores range from 0 for no abnormalities to a maximum score of 25, measuring the presence and extent of key morphologic features of bronchiectasis.
At three-monthly intervals serum samples and samples of spontaneously expectorated sputum were collected and stored at -70 oC. In the current study samples obtained from patients on azithromycin were included after unblinding the study data and reporting the clinical outcome of the study [7]. Samples at 3, 6, 9 and 12 months from start of treatment and 3 months after treatment discontinuation were used. In case of a missing sample at one of these visits, samples from directly previous or subsequent visits were used. Azithromycin was quantified in serum and in sputum using liquid chromatography, triple quad tandem mass spectrometry (LCMS/MS Agilent Technologies) and 13CD3 azithromycin as internal standard. Serum and sputum samples were kept at -70 0C until quantification. Azithromycin proved stable under these conditions. Before quantification sputum samples were homogenised by vortexing after addition of glass pearls. After addition of the internal standard quantification was performed in duplo. The method proved linear between 0094 and 18.9 mg/L in sputum and between: 0.0189 and 0.944 mg/L in serum. The limit of quantification was 0.1 mg/L in sputum and 0.02 mg/L in serum with a reproducibility of 1.4% in sputum (at 0.472 mg/L and 9.44 mg/L) and between 2.8% (at 0.028 mg/l) and 7.4% (at 0.472 mg/l) in serum.
Statistics
Comparisons of parameters between groups were calculated with a t test if normally distributed and with a Mann- Whitney U test if not.
When analysing the relation between azithromycin levels and clinical endpoints, we started by calculating Crohnbach’s alpha including measurements at 3, 6, 9 and 12 months for azithromycin serum and sputum levels in order to ascertain if it was justified to calculate means over time, accepting Crohnbach’s alpha >0.7 as sufficient. Change in clinical endpoint during one year of treatment was expressed by delta’s (measurement at 12 months minus baseline). The relationship between azithromycin concentrations and clinical endpoints was explored dually by calculating both Pearson’s correlation coefficient and performing linear regression for each variable. When calculating Pearson’s correlation coefficient r (p), r ≥ 0.7 was interpreted as indicating very strong correlation, 0.4- 0.69 as strong correlation, 0.3- 0.39 as moderate correlation, 0.2-0.29 as weak correlation and < 0.2 as no or negligible correlation. P < 0.05 was considered statistically significant. SPSS version 20 (SPSS inc.) was available for statistical analysis.
A total of 83 sputum samples from 31 patients were available for analysis. The percentage of patients able to produce spontaneous sputum decreased from 51% at baseline to 23% after one year of AZM treatment and increased to 47% after treatment discontinuation, which indicates a treatment effect of azithromycin. Serum samples were available for all AZM-treated patients (n= 43), yielding 151 serum samples for analysis. Baseline patient characteristics are described in (Table 1).
| Table 1: Baseline patient characteristics (n=43). | |
| Age (years, SD) | 59.9 (12.3) |
| Female sex (No,%) | 25 (63) |
| Body mass index | 23.0 (3.4) |
| Smoker | |
| Current | 1 (2) |
| Former | 19 (44) |
| Aetiology of bronchiectasis:* | |
| Post infectious | 15 (35) |
| Idiopathic | 12 (28) |
| Asthma | 7 (16) |
| Auto-immune disease | 3 (7) |
| Common variable immune disorder (CVID) | 1 (2) |
| Primary ciliary dyskinesia (PCD) | 1 (2) |
| Yellow Nail Syndrome | 0 |
| Aspiration | 1 (2) |
| Mechanical obstruction | 1 (2) |
| Allergic bronchopulmonary aspergillosis | 1 (2) |
| Alpha-1- antitrypsin deficiency | 1 (2) |
| No of exacerbations in year before study entry (median, IQR) | 4.0 (3-9) |
| HRCT score | 9.0 (3.0) |
| SGRQ total score | 40.6 (19.4) |
| LRTI-VAS total score | 17.5 (10) |
| Abnormalities on auscultation: | |
| Crackles | 20 (47) |
| Rhonchi | 8 (19) |
| Wheezing | 7 (16) |
| Dullness | 0 |
| CRP (mmol/l) (median, IQR) | 5.0 (2- 11,3) |
| WBC count (x109/L) | 8.1 (2.7) |
| Percent predicted FEV1 | 77.7 (24.4) |
| Percent predicted FVC | 91.9 (24.4) |
| Baseline sputum microbiology: | |
| Haemophilus influenzae | 13 (30) |
| Staphylococcus aureus | 4 (9) |
| Pseudomonas aeruginosa | 6 (14) |
| Treatment previous to study entry: | |
| Inhaled corticosteroids‡ | 38 (88.4) |
| Long-acting β-agonist‡ | 34 (79) |
| Oral corticosteroids‡ | 4 (9) |
| Inhaled antibiotics‡ | 0 (0) |
| Longterm oral antibiotic treatment‡ | 4 (9) |
| Airway clearance techniques: § | |
| Daily | 11 (26) |
| Weekly | 3 (7) |
| During exacerbation | 4 (9) |
| Data are n(%) or mean (SD) unless otherwise indicated. FEV1 = forced expiratory volume in 1 sec. FVC = forced vital capacity. SGRQ= St George’s respiratory questionnaire. LRTI-VAS= lower respiratory tract infection- visual analogue score. * As described by the treating pulmonary physician. † patient reported hearing impairment; ‡ Treatment started before study entry and continued during the study period. § Any technique taught by a physiotherapist and performed by the patient in order to evacuate sputum. | |
Crohnbach’s alpha for measurements of AZM levels at 3, 6, 9 and 12 months was 0.71 for serum AZM levels and 0.74 for sputum AZM, indicating sufficient correlation between different measurements and allowing us to calculate means.
Clinical endpoints
A mean number of 4.47 (SD 1.55) exacerbations for each patient were noted during the year before start of study as compared to 0.84 (SD 1.13) while receiving azithromycin (p<0.001). During one year of azithromycin treatment changes in other clinical parameters were as follows:
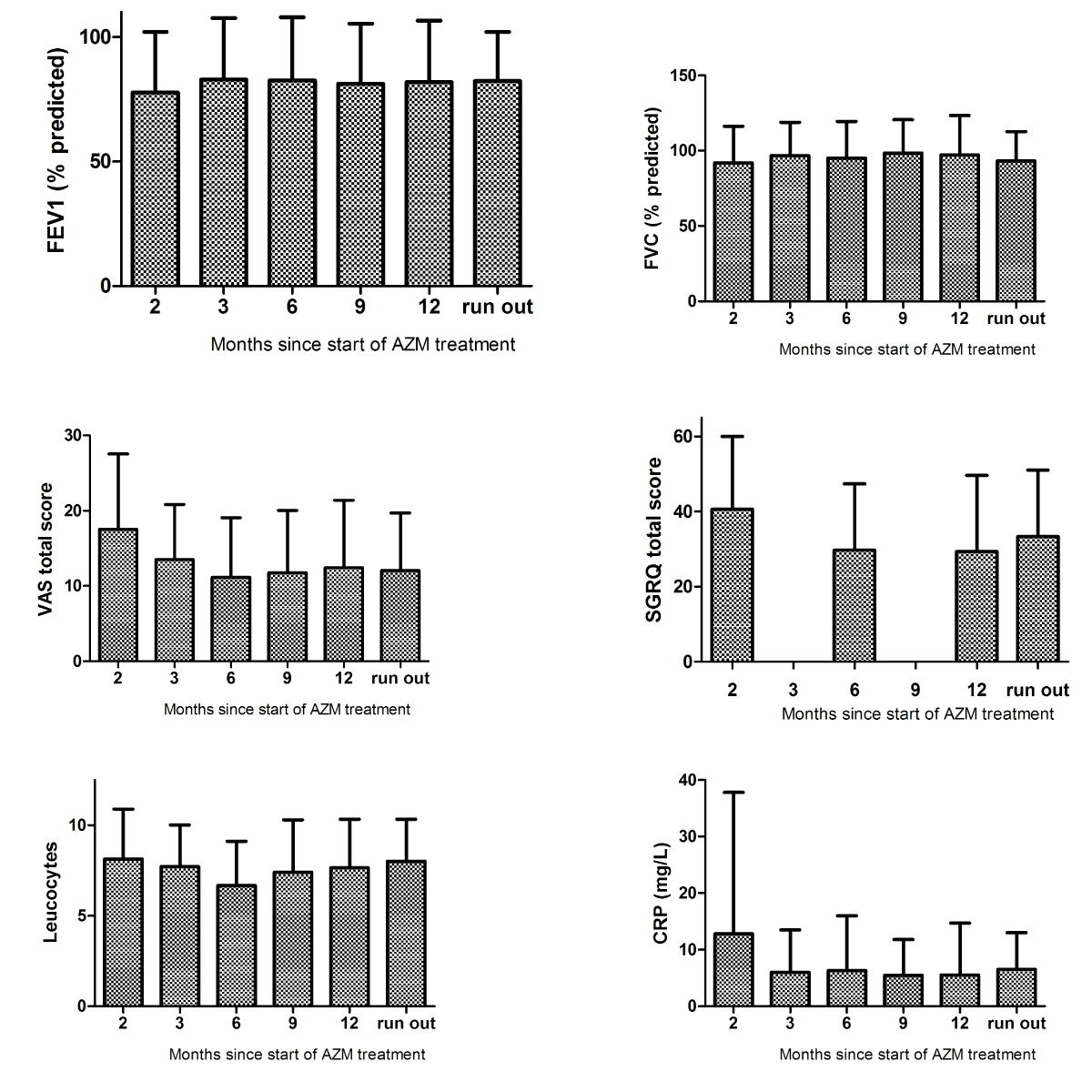
FEV1 and FVC at baseline were 77.7 (SD 24.4) and 91.9 (SD 24.4) % of predicted as compared to 81.9 (SD 24.6) and 97.2 (SD 26.1) % pred. at 12 months. VAS total score decreased from 17.5 (SD 10.0) at baseline to 12.4 (SD 8.95) at 12 months and SGRQ total score declined from 40.6 (SD 19.4) to 29.3 (SD 20.4). CRP and leukocytes at baseline were 12.8 (SD 25.0) and 8.14 (SD 2.76) respectively as compared to 5.5 (SD 9.2) and 7.7 (SD 2.7) at end of treatment (e-figure 1).
e-figure:
Azithromycin serum- and sputum levels
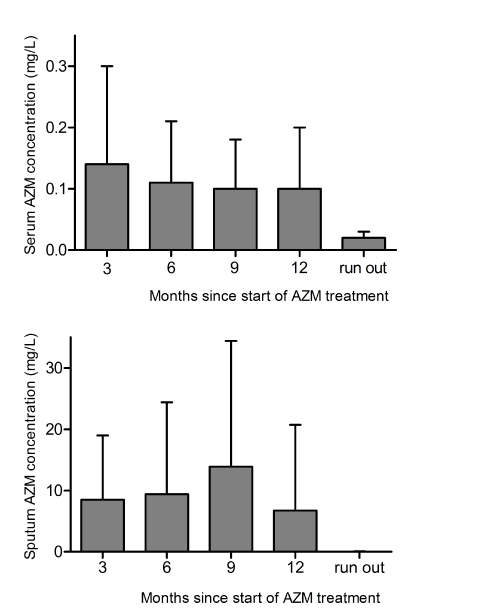
Mean azithromycin concentration for all visits was 7.57 mg/L (SD 9.49 mg/L) in sputum and 0.11 mg/L (SD 0.085 mg/L) in serum (Figure 1 and Table 2).
Figure 1: Mean AZM concentrations in sputum and serum during –and three months after discontinuation of- AZM 250 mg OD maintenance treatment. AZM: azithromycin. Error bars represent standard deviations.
| Table 2: Mean azithromycin concentrations (mg/L) in sputum and serum during one year of maintenance treatment (azithromycin 250 mg once daily). | ||||
| Months from start of treatment | No. of patients | Mean serum concentration (SD) | No. of patients | Mean sputum concentration (SD) |
| 3 | 43 | 0.14 (0.16) | 22 | 8.49 (10.5) |
| 6 | 43 | 0.11 (0.10) | 27 | 9.41 (15) |
| 9 | 43 | 0.10 (0.08) | 24 | 13.9 (20.5) |
| 12 | 43 | 0.10 (0.10) | 10 | 6.71 (14) |
| 3 months after treatment discontinuation | 43 | 0.002 (0.014) | 20 | 0.02 (0.09) |
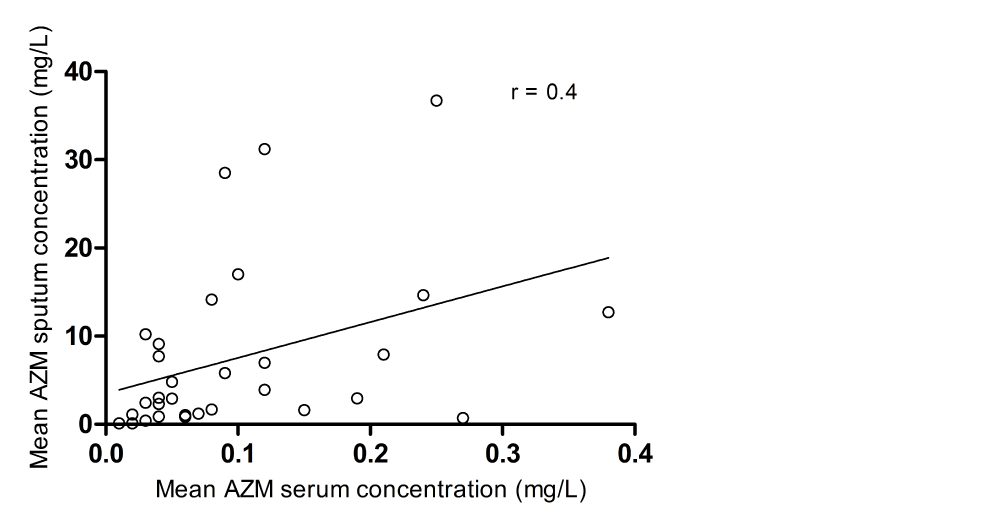
There was moderate correlation between sputum and serum levels in this patient group (r=0.4 (Pearson)) (Figure 2). Three months after discontinuation of treatment sputum samples from all but one patient and serum samples from all but one other patient were negative for azithromycin (sputum <0.1 mg/L, serum <0.02 mg/L). Correlations between AZM concentration in serum and sputum and change of clinical endpoints such as lung function, HRCT score and exacerbation frequency are shown in table 3. Sputum AZM levels showed moderate-good correlation with change of VAS total score, leukocyte count and CRP levels, but only the correlation between sputum AZM and CRP reached statistical significance when performing regression analysis (p=0.001). No correlation was found between AZM serum concentrations and all clinical endpoints (p>0.05).
Figure 2: Correlation between mean azithromycin sputum- and serum levels in 43 patients receiving one year of maintenance treatment (azithromycin 250 mg once daily). r represents Pearson’s correlation coefficient..
| Table 3: Correlations between mean AZM serum and sputum levels and change of clinical endpoints* during one year of maintenance treatment. | ||||||
| Serum | Sputum | |||||
| n | r (p) | Regression coefficient (SD) | n | r (p) | Regression coefficient (SD) | |
| Exacerbation frequency | 43 | 0.02 (0.9) | 0.4 (3.1) | 31 | 0.1 (0.6) | 0.02 (0.03) |
| FEV1 (percent predicted) | 37 | -0.03 (0.85) | -3,3 (17) | 26 | 0.2 (0.2) | 0.2 (0.2) |
| FVC (percent predicted) | 36 | 0.09 (0.61) | 13.8 (26.9) | 25 | 0.4 (0.08) | 0.5 (0.3) |
| VAS total score | 34 | -0.1 (0.5) | -16.5 (24) | 24 | -0.4 (0.07) | -0.5 (0.3) |
| SGRQ total score | 36 | 0.2 (0.3) | 38.6 (33.1) | 25 | 0.04 (0.86) | 0.07 (0.4) |
| CRP | 40 | -0.2 (0.3) | -41.7 (41.8) | 28 | -0.6 (0.001) | -1.3 (0.4) |
| Leukocyte count | 40 | -0.03 (0.8) | -1.2 (5.8) | 28 | -0.4 (0.07) | -0.1 (0.06) |
| * Δ visit 6 (12 months) – visit 2 (baseline). | ||||||
When comparing AZM serum- and sputum levels in different patient groups (classified by smoking habit, etiology (idiopathic/ post infectious/ other diagnosis) or gender, no between group-difference was found. In addition, no correlation between AZM levels and age or weight existed.
Dose level
Mean body weight of the participants was 66,6 kg (SD 12.8) and a mean dose-level of azithromycin of 26,3 mg/kg bodyweight/week (bw/wk) (range 18.8 to 39.8 mg/kg bw/ wk) was calculated. No or very weak correlation was found between dose level and change in exacerbation frequency (r=0.14), FEV1 (r=0.21), FVC (r=0.23),SGRQ- and LRTI-VAS scores (r=-0.05 and -0.1 respectively) and HRCT-scores (r=-0.04). Only weak correlation was found between dose level and AZM sputum levels (r=0.3) and no correlation between dose level and AZM serum levels.
Side effects
During AZM treatment 23 of 43 (53%) patients reported any side effects, mostly mild gastro-intestinal complaints. When comparing dose levels in patients with or without any side effects, no significant difference between groups was found (p=0.57). When comparing AZM serum and sputum concentrations in patients with or without any side effects, no significant difference between groups was found (p=0.85 and 0.84 respectively).
In this study we quantified AZM concentrations in serum and sputum of bronchiectasis patients receiving AZM maintenance treatment (250 mg OD). To our knowledge this is the first study to report this type of data on AZM maintenance treatment in a large group of patients with bronchiectasis without CF. Much more is known on kinetics of AZM in CF patients, for whom it is often presumed that bioavailability and pharmacokinetics of azithromycin and possibly other drugs differ from non-CF patients. However, already in 2005 Beringer et al. [14] reported that –in comparison with healthy volunteers- the bioavailability, absorption rate and pharmacokinetics of single dosages of AZM in CF patients taking pancreatic enzyme suppletions were no different.
We found azithromycin sputum concentrations ranging from 6.71 mg/L to 13.9 mg/L, about 70 times higher than in serum. Reports in CF patients taking either 500 OD or 1000 mg once weekly describe higher sputum concentrations (26.6 mg/L (SD15.6) at 500 mg OD and 9.6 (SD7.1) at 1000 mg weekly), but the level of accumulation is comparable [15].
Only moderate correlation was found between serum and sputum concentrations of AZM. This is in concordance with the results of Wilms et al. [16] 20 who also failed to demonstrate a strong relationship between sputum and blood concentrations in CF patients. Their hypothesis that sputum concentrations might be influenced by the availability of neutrophils in the lungs and the amount of sputum that is produced, might also apply to non-CF bronchiectasis. In this view, neutrophils, with their high intracellular level of AZM would act as so-called ‘vehicles’ for AZM transportation, delivering relatively large amounts of antibiotics to the site of inflammation. This is further supported by the finding of higher concentrations of azithromycin in infected versus uninfected tissue in a mouse thigh infection model and in inflamed versus non inflamed blisters in humans [17,18].This might especially be true for bronchiectasis, because its clinical course is characterized by periods with usually mild chronic complaints, interspersed with infectious exacerbations. During stable disease, and even more so during an exacerbation, markedly raised numbers of neutrophils are found in the airways of bronchiectasis patients, not necessarily accompanied by raised systemic inflammation markers. This airway-predominant inflammation may in part account for the differences between AZM serum- and sputum levels in this study.
As reported earlier, favourable changes in clinical parameters were noted during one year of azithromycin treatment [7]. Apart from an evident reduction of infectious exacerbations, a small improvement in lung function was seen together with an improvement of quality of life as measured by SGRQ. In addition, symptoms and inflammatory parameters were reduced. No earlier studies have reported the relationship between clinical efficacy and the individual exposure to azithromycin during maintenance treatment. Since AZM shows multiple pharmacodynamic effects of which the contribution to the clinical efficacy has not been fully understood, a clear concentration-exposure relationship was not expected. However, in earlier reports, macrolides have been described to suppress sputum production through inhibition of chloride secretion by airway epithelial cells [19]. Tagaya et al. [20], described a dose dependent effect of erythromycin, on chloride diffusion in an animal model. In our study the exposure-effect relationship was less distinct; although changes in FVC, VAS total score, CRP level and leukocyte count showed moderate-good correlation to AZM sputum levels, only the correlation with CRP-level reached statistical significance. The current study failed to demonstrate any significant correlation between response parameters and serum AZM concentrations.
In the current study a standard dosing regimen of AZM 250 mg daily resulted in a wide range of azithromycin dose levels (dose per kg bodyweight). Dose level did not appear to influence systemic exposure to the study drug since correlation between dose level and AZM-levels in serum and sputum was moderate at best. In CF patients azithromycin maintenance therapy leads intra-individually, to concentrations in bronchial secretion approximately linearly related to the oral dose and irrespective of the azithromycin dosing frequency and interval. The inter-individual variability in drug concentrations is therefore likely due to patient-specific parameters. However, when analysing the available data on age, weight, etiology, smoking status or gender in relation to AZM levels, no such parameter was identified. Other factors, such as bioavailability, therapy adherence, number of neutrophils in sputum and sputum kinetics might importantly influence local and systemic drug levels.
An unexpected finding in the current study is the absence of a relation between the occurrence of side effects and AZM concentrations or dose level. In the past decade, several authors reported an increased incidence of adverse effects with larger dosages of macrolides or higher AZM serum concentrations in CF or Mycobacterium avium complex (MAC)-disease [21,22]. A similar dose-dependent occurrence of side effects is observed when comparing adverse events in the BAT trial and the EMBRACE trial by Wong and colleagues.9 During treatment with AZM 250 mg daily, 40% of participants in the BAT trial experienced gastro-intestinal side effects as compared to 27% in the latter trial using 500 mg AZM thrice weekly [7,23].
In the current study, clinical improvement appears to be unrelated to AZM dose level, therefore one could speculate if the dose of 250mg/day chosen in the BAT trial might not be unnecessarily high in patients with lower body weight. In these patients a dosing regimen of 250 every other day - as is already frequently used in pulmonary clinics – might be sufficient. To date, no randomised trials comparing different dosing regimens are available. For patients with cystic fibrosis one of the current authors recently proposed a dose advice of AZM 22-30 mg/kg/wk, based on efficacy in clinical trials.10 In the current trial, the lowest dose level inducing a clinically relevant response was 18,85 which corresponds to a daily dose of AZM of approximately 150 mg for patients with a body weight between 55 and 60 kilos.
Although the current study provides interesting and new information on the relations between AZM dose level, serum- and sputum concentration and clinical effect parameters, the authors wish to point out a number of weaknesses, mostly related to the study design.
The original BAT trial was not designed to measure pharmacokinetic parameters, which means that no information was available about the exact timing of sputum expectoration or blood sampling in relation to drug ingestion. Especially the serum AZM levels have to be interpreted with caution, since AZM concentrations in blood show a distinct pattern characterized by a peak within hours after ingestion, followed by quick distribution into the tissue [16]. Therefore, the timing of blood sampling will importantly contribute to variations in AZM levels. This methodological problem may also be one of the reasons that no correlation was found between AZM serum levels and other parameters in the current study. AZM sputum concentrations are more robust, since earlier studies showed that accumulation of AZM in bronchial secretions still occurs after 5 days of treatment, reaching stable values in about 1 month of treatment, yielding stable values throughout time and small intra-individual variations [24].
Second, the availability of sputum samples gradually decreased during AZM treatment. Results for visit 9 and 12 might therefore be less robust when data from sputum analysis are involved. Finally when quantifying the total azithromycin concentration in sputum we were not able to distinguish between intra- and extracellularly, bound and unbound azithromycin [25].
In conclusion: one year of AZM maintenance treatment resulted in high levels of sputum AZM as opposed to serum levels which were about 70 times lower. Higher sputum concentrations of AZM only coincided with a reduction of serum CRP, but showed poor correlation to other response parameters. Contrary to findings in the literature and our own clinical experience, we failed to demonstrate a relation between adverse events and AZM concentrations or dose level. Considering the favourable response to treatment in patients with a relatively low dose level of AZM, it may be justified to apply reduced dosage regimens for patients with low body weight [26].
The authors thank H. Trumpie, Central Hospital Pharmacy The Hague who performed the analysis of AZM and T. van der Ploeg, Foreest Medical School Alkmaar Medical Centre for his help with the statistical analysis.
Funding information
This study was supported by an unrestricted research grant from Teva Netherlands (NL/RESP/14/0001). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Transparency declarations
J. Altenburg contributed to designing the study, performed the data collection, analysis and interpretation, and drafted the manuscript.
E.B Wilms contributed to designing the study, supervised the analysis of AZM and revised the manuscript critically for important intellectual content.
W.G. Boersma contributed to designing the study and revised the manuscript critically for important.
All authors had full access to the data and can take responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version of the manuscript.
- Van BF, Tulkens PM. Macrolides: pharmacokinetics and pharmacodynamics. Int J Antimicrob Agents. 2001; 18: S17-S23. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/11574190
- Di PA, Barbara C, Chella A, Angeletti CA, Del Tacca M. Pharmacokinetics of azithromycin in lung tissue, bronchial washing, and plasma in patients given multiple oral doses of 500 and 1000 mg daily. Pharmacol Res. 2002; 46: 545-550. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/12457629
- Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA Jr, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011; 365: 689-698. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/21864166
- Uzun S, Djamin RS, Kluytmans JA, Mulder PG, van't Veer NE, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014; 2: 361-368. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/24746000
- Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013; 68: 322-329. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/23291349
- Chalmers JD, Elborn JS. Reclaiming the name 'bronchiectasis'. Thorax. 2015; 70: 399-400. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/25791834
- Altenburg J, de Graaff CS, Stienstra Y, Sloos JH, van Haren EH, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013; 309: 1251-1259. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/23532241
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993; 16: 5-40. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/8499054
- Altenburg J, Wortel K, de Graaff CS, van der Werf TS, Boersma WG. Validation of a visual analogue score (LRTI-VAS) in non-CF bronchiectasis. Clin Respir J. 2016; 10: 168-175. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/25103370
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD. 2005; 2: 75-79. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/17136966
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis. 1992; 145: 1321-1327. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/1595997
- Wilson CB, Jones PW, O'Leary CJ, Cole PJ, Wilson R. Validation of the St. George's Respiratory Questionnaire in bronchiectasis. Am J Respir Crit Care Med. 1997; 156: 536-541. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/9279236
- Bhalla M, Turcios N, Aponte V, Jenkins M, Leitman BS, et al. Cystic fibrosis: scoring system with thin-section CT. Radiology. 1991; 179: 783-788. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/2027992
- Beringer P, Huynh KM, Kriengkauykiat J, Bi L, Hoem N, et al. Absolute bioavailability and intracellular pharmacokinetics of azithromycin in patients with cystic fibrosis. Antimicrob Agents Chemother. 2005; 49: 5013-5017. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/16304166
- Wilms EB, Touw DJ, Heijerman HG. Pharmacokinetics and sputum penetration of azithromycin during once weekly dosing in cystic fibrosis patients. J Cyst Fibros. 2008; 7: 79-84. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/17599845
- Wilms EB, Touw DJ, Heijerman HG. Pharmacokinetics of azithromycin in plasma, blood, polymorphonuclear neutrophils and sputum during long-term therapy in patients with cystic fibrosis. Ther Drug Monit. 2006; 28: 219-225. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/16628134
- Carbon C. Clinical relevance of intracellular and extracellular concentrations of macrolides. Infection. 1995; 23: S10-S14. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/7782109
- Liu P, Allaudeen H, Chandra R, Phillips K, Jungnik A, et al. Comparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single-dose extended-release regimen versus a 3-day immediate-release regimen. Antimicrob Agents Chemother. 2007; 51: 103-109. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/17060516
- Tamaoki J, Isono K, Sakai N, Kanemura T, Konno K. Erythromycin inhibits Cl secretion across canine tracheal epithelial cells. Eur Respir J. 1992; 5: 234-238. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/1559589
- Tagaya E, Tamaoki J, Kondo M, Nagai A. Effect of a short course of clarithromycin therapy on sputum production in patients with chronic airway hypersecretion. Chest. 2002; 122: 213-218. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/12114361
- McCormack J, Bell S, Senini S, Walmsley K, Patel K, et al. Daily versus weekly azithromycin in cystic fibrosis patients. Eur Respir J. 2007; 30: 487-495. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/17537764
- Brown BA, Griffith DE, Girard W, Levin J, Wallace RJ Jr. Relationship of adverse events to serum drug levels in patients receiving high-dose azithromycin for mycobacterial lung disease. Clin Infect Dis. 1997; 24: 958-964. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/9142801
- Wong C, Jayaram L, Karalus N, Eaton T, Tong C, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012; 380: 660-667. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/22901887
- Wilms EB, Touw DJ, Heijerman HG, van der Ent CK. Azithromycin maintenance therapy in patients with cystic fibrosis: a dose advice based on a review of pharmacokinetics, efficacy, and side effects. Pediatr Pulmonol. 2012; 47: 658-665. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/22684985
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. Standardisation of spirometry. Eur Respir J. 2005; 26: 319-338. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/16055882
- Serisier DJ, Martin ML, McGuckin MA, Lourie R, Chen AC, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA. 2013; 309: 1260-1267. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/23532242