More Information
Submitted: 22 January 2020 | Approved: 03 February 2020 | Published: 04 February 2020
How to cite this article: Batain F, Crescencio K, Alves T, Souza JF, Amaral V, et al. Medicinal plant extract associated with bacterial cellulose membrane: Antibacterial activity and physicochemical properties. Arch Pharm Pharma Sci. 2020; 4: 013-020.
DOI: 10.29328/journal.apps.1001022
ORCiD: orcid.org/0000-0003-3618-8415
ORCiD: orcid.org/0000-0002-3684-3275
Copyright License: © 2020 Batain F, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Bacterial cellulose membranes; Aloe vera. Matricaria recutita. Calendula officinalis; Burn; Wound healing
Medicinal plant extract associated with bacterial cellulose membrane: Antibacterial activity and physicochemical properties
Fernando Batain1, Kessi Crescencio1, Thais Alves1, Juliana Ferreira Souza1, Venâncio Amaral1, Juliana Castro2, Carolina Santos3, Angela Jozala4, Luciane Lopes5* and Marco Chaud1*
1Laboratory of Biomaterial and Nanotechnology (LaBNUS). University of Sorocaba, Brazil
2PPGCF – Post-Graduate Program in Pharmaceutical Sciences of the University of Sorocaba, Sorocaba/SP, Brazil
3College of Pharmacy. University of Sorocaba, Brazil
4Laboratory of Industrial Microbiology and Fermentation Process, University of Sorocaba, Brazil
5Seriema - Evidence Service for Monitoring and Evaluation, University of Sorocaba, Sorocaba, Brazil
*Address for Correspondence: Marco Chaud, Laboratory of Biomaterial and Nanotechnology (LaBNUS), University of Sorocaba, Brazil, Tel: 55+ (15) 98153-6692; Email: [email protected]
*Address for Correspondence: Luciane Lopes, Seriema - Evidence Service for Monitoring and Evaluation, University of Sorocaba, Sorocaba, Brazil, Tel: +55 (15) 2101-7104; Email: [email protected]
Burns injuries induce a state of immunodepression that predisposes to a bacterial infectious complication that leads to several comorbid diseases and high mortality rate. Previous studies about anti-inflammatory, antimicrobial and antioxidant properties of Aloe vera (L.) Burm., Calendula officinalis L.and Matricaria recutita L. are acknowledge by antimicrobial effects.
Previous studies about anti-inflammatory, antimicrobial and antioxidant properties of Aloe vera (L.) Burm., Calendula officinalis L. and Matricaria recutita L. are knowledge by antimicrobial effects. Bacterial cellulose membrane (nature BCM) is a potential carrier as a drug delivery system in the wound and burn treatment. The present study aimed to evaluate the antibacterial activity of extracts of A. vera, C. officinalis, and M. recutita incorporated in BCM against bacterial strains commonly present in wound and burns. The agar-dilution susceptibility testing was used to determine the minimum inhibitory concentration (MIC) for S. aureus, E. coli, and P. aeruginosa. The standardized extracts of A. vera, M. recutita, and C. officinalis were, respectively, used at 3.25% of total polysaccharides, 1% of apigenin 7-O-glucoside and 0.084% of total flavonoids expressed in quercetin. The BCM incorporated with A. vera extract was efficient to prevent the growth of P. aeruginosa and S. aureus. BCM loaded with C. officinalis inhibited the growth of S. aureus. The BCM loaded with A. vera and C. officinalis extract showed better antibacterial activities against P. aeruginosa and S. aureus and, consequently, properties to prevent infectious disease in the wound or burn caused by these bacteria.
Burns and wounds are injuries of the cutaneous tissue caused by heat, radiation, radioactivity, electricity, skinned, or chemicals. Both wounds and burns are accidentals with a high occurrence level. In the world, approximately 265,000 deaths occur annually, which represents a severe public health problem [1]. Studies have been reporting that skin injuries are related to microbial colonization, suppressing the immune function predisposing the people to infectious diseases [2]. Hassen, et al. [3], reported that the most commonly isolated bacteria in burn children were methicillin-susceptible Staphylococcus aureus (MSSA, 57.7%), Pseudomonas aeruginosa (35.9%), and Enterobacter cloacae (26.9%) wild types [3].
Aloe vera (L.) Burm. f. (A. vera), Calendula officinalis L. (C. officinalis) and Matricaria recutita L. (M. recutita) are indicated, respectively, for treatment of first and second-degree burns, acting a healing and anti-infectious properties for topical use [4,5]. M. recutita mentioned in the monographs of the World Health Organization, Micromedex, British National Formulary Herbal Medicines, and Normative Instruction No. 02/2014 of the National Sanitary Surveillance Agency in the treatment of injuries of the epidermis and mucous membrane.
In a pre-clinical study involving rats as an animal model for a topical evaluation of medicinal plants, it was found that M. recutita, compared to corticosteroids, promotes a faster healing process [6]. Erythema and inflammation are late acute side effects induced by radiation-therapy. Hu, et al. [7], and Schneider, et al. [8], showed that C. officinallis extract reduced both damages of skin [7,8].
Biopolymers with regenerative properties have been studied as carriers of drugs to improve the therapeutic properties and to accelerate the process of damaged tissue regeneration [9]. BCM from the biosynthesis of microorganisms is among the most promising biopolymers for drug delivery carriers [10,11] due to its environmentally friendly, nanosized crystal, hydrophilicity, porosity, wide surface area, tasteless, and odourless properties, which can be monitored during the production process [12,13].
Studies on standardized plant extracts obtained from A. vera, C. officinalis and M. recutita and many others have been extensively reported in the scientific research papers [14-17] and review works on this subject [18].
The antibacterial activity of medicinal plants in solution, gel, toothpaste, creams, tablets, nanoparticle systems, and other dosage forms have been evaluated and many products are commercially disponible. However, the medicinal plant extracts incorporated into BCMs were not reported so far. Then, this work is a pioneer research effort to join the biological and physicalchemical properties of BCM with standardized medicinal extracts of C. officinalis, A. vera, and M. recutita, against bacterial strains S. aureus, E. coli, and P. aeruginosa commonly present in wound and burns.
Standard plants extracts
The plants’ extracts of Aloe vera, Matricaria recutita (Group Center Flora - Lot: 271115.2850) and Calendula officinalis (Bio Tae Plant Extracts - Lot: 025085) were standardized according to Normative Instructions 05/2008 and 02/2014 [4,5]. The extracts used in this study showed markers of total polysaccharides for Aloe vera (3.25%), apigenin 7-O-glucoside for Matricaria recutita (1%), and total flavonoids expressed in hyperosides for Calendula officinalis (0.084%).
Microorganisms strains
Escherichia coli (American Type Culture Collection) ATCC 25922, Pseudomonas aeruginosa ATCC 9721, and Staphylococcus aureus ATCC 10390 were used to determine the Minimum Inhibitory Concentration (MIC). Cefar Diagnostica Ltda Brazil provided the bacteria utilized to evaluate the antimicrobial activity by agar diffusion susceptibility method. The reports showed biochemical compatibility with all strains that had been used on MIC assays.
Inoculum preparation
To inoculum preparation the bacteria were resuspended in standard culture medium and maintained at 37°C for 24 h in the BOD micro-processed oven (M502, Fanem, SP. Brazil). For the evaluation of MIC, the inoculum concentration was standardized at a final concentration of 106 CFU.mL-1 as described by Cavalieri, et al. 2005 [19]. The inoculum used in the agar diffusion sensitivity test was standardized by McFarland scale.
The results obtained in the visual comparation, 1.108 – 2.108 CFU.mL-1 was confirmed by the turbidimetric method (Shimadzu, Multispec 1501, Quito, Japan), which absorbance was measured with optical density between 0.08 and 0.1 at λ 625 nm [20].
Minimum inhibitory concentration determination
The MIC determination was done using by serial microdilution method in the 96-well sterile microplate. For microdilution, a sample of the 200 μL (100mg.mL-1) of each plant’s extract was, respectively, put into 1th well; into 2nd to 12th well were distributed 100 μL of culture medium. The serial microdilution was performed step by step for each plant extract to obtain an inoculum dilution correspond at 106 CFU.mL-1 from 2nd to 10th well of each series, which were incubated at 37oC during 24h. The 11th and 12th well of each series were, respectively, used to the negative and positive control.
Preparation of bacterial vellulose membrane
The BCMs were obtained from the culture of Gluconacetobacter xylinus in Hestrin & Schramm medium (glucose 20 g. L-1, bacteriological peptone 5.0 g. L-1, yeast extract 5.0 g. L-1, sodium phosphate 2.7 g. L-1, citric acid monohydrate 1.5 g. L-1). To obtain a membrane with nearly 2 mm of thickness was used 1 mL of inoculum of G. xylinus (106 CFU.mL-1) into a well with 3 cm of diameter.
The plates were closed and kept at 30oC in the static culture medium for four days. BCMs obtained were removed of the wells and washed by immersed in an aqueous solution with sodium dodecyl sulphate (SDS) 2% for 24h. Following the BCM were rinsed with purified water to remove SDS, and immersed in NaOH 1M, maintained at 60oC, under constant stirring (50 rpm) for 1.5 h. After this time, the BCM was washed in purified water until pH 6.8-7.0), and kept in physiological solution, and autoclaved at 121oC, 15 min. The membranes were kept in physiological solution and stored at 4oC [21].
Extracts loading rate into bacterial cellulose membrane
The loading rate of plants extracts was performed as described by Shezad, et al. 2010, with modifications [22]. Briefly, the BCM previously dehydrated were submerged into 1 mL of the aqueous solution of the A. vera, M. recutita (0.5 mg. mL-1), and C. officinalis (0.8 mg. mL-1) and remained under gentle stirring 50 rpm at 25°C for up to saturation equilibrium. The saturation point was determined by gravimetry, and the maximum necessary time to swelling of the BCM was recorded. The test was performed six time (n = 6). The incorporation capacity was determined according to equation 1, where: iw = initial weight e fw = final weight.
Antimicrobial activity evaluation
The BCM, previously dehydrated, were incorporated with 1.0 mL of aqueous solutions of the plants’ extracts and were placed on the surface of TSA (trypticase soy agar) culture medium with a bacterial strain of E. coli, P. aeruginosa, or S. aureus.
As negative control was added to disk diffusion imbibed in sterile water (inert disks), and as a positive control, diffusion disks containing 5.0 µL of a solution of the penicillin 10.000 UI. mL-1 and streptomycin 10 mg. mL-1 were prepared.
To evaluate the effect of BCM in the delivery of the extracts were prepared disks of the cellulose paper with 30 µL of each plant extract in the concentrations previously cited. The plates were incubated in an oven at 37oC, 24h. The test was performed in duplicate.
Fourier transformed infrared spectroscopy
This spectroscopic analysis (FTIR IRAffinity-1S- Shimadzu, Kyoto. Japan) was performed by Attenuated Total Reflectance (ATR) in transmittance mode, in order to characterize and identify chemical groups in BCM samples. The spectrograms were obtained in a range of wavenumber between 4,000 – 600 cm-1, with 128 scan and resolution of 4 cm-1.
Differential scanning calorimetry
The BCM thermograms were obtained by DSC 60 (Thermal analyser TA 60W-Shimadzu, Kyoto, Japan). One warming cycle was applied with temperature range between 25 and 350oC, in the heating rate of 10oC. min-1, under N2 flow (50 mL. min-1).
Mucoadhesive properties
The BCM mucoadhesive property with or without plant extract was evaluated on mucin discs using on texture analyser (TAXTPlus Texture analyser - Stable Micro Systems, UK), following the protocol developed and validated at the Laboratory of Biomaterials and Nanotechnology of the University of Sorocaba (LaBNUS). The mucin discs were obtained by compression (Lemaq, Rotary Tablet Press Machine, Mini Express LM-D8, Diadema, BR), using flat punctures, 8 mm diameter cylindrical die, and 8-ton compression load. The mucin discs had 110 mg, 8 mm diameter, and 0.2 mm of thickness. The mucin discs were hydrated for the test performed, the excess of water of discs surface was removed with absorbent paper. Mucin discs were bound with double-sided tape on the underside of the analytical probe.
One sample of each BCM was transferred for a jacketed glass Becker with temperature adjusted at 37oC, and the disc was compressed onto the BCM surface with a force of 0.1 N, apically → basally directed. The contact time of disc with the sample was standardized for 300 seconds. The basally → apically displacement of the analytical probe was at 10 mm.s-1. The force required to separate the mucin disc from the surface of BCM was defined from a time (s) x force (N) relationship. All measurements were performed in triplicate.
Statistical analysis
To determine the statistical difference of the mechanical and mucoadhesive properties were used analysis of variance (ANOVA) followed by Tukey test for multiple comparison of means and 95% confidence interval (p < 0.05).
Minimum inhibitory concentration of extracts
Inhibitory bacterial activity of A. vera, M. recutita, and C. officinalis extracts in BCM were evaluated against Gram-positive and Gram-negative bacteria. The MIC results (Table 1) showed resistant E. coli activity for BCM loaded with C. officinalis. BCM loaded with A. vera extract was more effective against S. aureus than against P. aeruginosa and E. coli. This result can be explained by the phytochemical composition of A. vera (polysaccharides, tannins, steroids, organic acidic and saponin between others) to contain antimicrobial and antioxidant activities [23,24].
| Table 1: Minimum inhibitory concentration of antimicrobial activity in A. vera, M. recutita and C. officinalis extracts. | |
| Microorganisms | Plants extracts (mg. mL-1) |
| A. vera M. recutita C. officinalis | |
| P. aeruginosa | 5 2,5 20 |
| S. aureus | 2,5 1,25 20 |
| E. coli | 10 10 NI* |
| *NI: No Inhibition | |
BCM loaded with M. recutita also showed more microbiological activity against S. aureus than P. aeruginosa and E. coli. However, when compared with A. vera the antimicrobial activity of M. recutita is 50% lower. Except the activity absence for C. officinalis, no difference between A. vera and M. recutita was found for strains of E. coli.
The major components of M. recutita was described as α-bisabolol oxide (38%), followed by camphene (9.11%), saponin (4.87%), limonene (6%), 1,8-cineole (7.12%), camphor (6.54%), and α-pinene (6%), where α-bisabolol oxide has been found to be the most active compounds (25). The bacterial inhibitory activity of BCM loaded C. officinalis had a lower inhibitory bacterial activity than A. vera and M. recutita.
BCM loading rate
The measure of loading rate is an essential parameter and is correlated with antimicrobial activity. The higher loading rate was found for A. vera (225%), M. recutita (268%), and C. officinalis (76%). The results analyses showed a selective chemical affinity for the different compounds; apigenin 7-O-glucoside (M. recutita), polysaccharides (A. vera), and hyperosides (Calendula officinalis). The chemical selectivity can be given by different factors, between them the water solubility and intermolecular interaction with cellulose [26]. The water solubility of the key compound, as well as 7-O-glucoside and hyperosides, is, respectively, 970 mg. L-1 (log P 0.44), and 2,782. 107 mg. L-1, (log P -0.11). The higher affinity can explain the highest water solubility of hyperosides. Consequently, there is more affinity by the aqueous medium than by BCM. The loading rate of polysaccharides can be justified by hydrogen-bonding interactions with cellulose [27].
Antimicrobial activity by agar diffusion
The absorbance unit at 625 nm obtained by the turbidimetric method were 0.084, 0.088, 0.085, respectively, for P. aeruginosa, S. aureus, and E. coli. These results show that the concentrations of the microorganisms in the culture medium were the same; then the results can be compared with each other and with the positive and negative controls.
Antibacterial activity of plant extract was measured (mm) by the diameter of inhibition zones and was recorded after 24h of incubation. The antagonistic action of BCM-extracts and BCM without extract were tested against the ATCC strains (n = 2). The results are shown in table 2. The bacterial growth absence in plates with culture medium (negative control) shows that it did not have extemporaneous contamination during assay time. On the other hand, the result for BCM showed that the membranes did not have antagonistic activity.
| Table 2: Bacterial sensibility of the strains S. aureus, E. coli, and P. aeruginosaagainst A. vera, C. officinalis, and M. recutita extracts in BCM, compared with negative control (BCM). Positive control (Penicillin 10.000 UI/mL + Streptomycin 10 mg/mL disks), and culture medium sterility control (CM). | ||||||
| Antibacterial activity by inhibition zone (mm) M ± SD | ||||||
| Microorganism | A.vera | C.officinalis | M.recutita | BCM† | PC‡ | CM* |
| P.aeruginosa | 17 ± 1.4 | NI | NI | NI | 16 ± 1.1 | (-) |
| S.aureus | 15 ± 0.7 | 17,5 ± 0.7 | NI | NI | 32 ± 1.1 | (-) |
| E.coli | NI | NI | NI | NI | 17 ± 0.6 | (-) |
| †BCM: Bacterial Cellulose Membrane without extract (Negative Control); ‡PC: Positive Control; *CM: Culture Medium; R: Resistant strain; NI: No Inhibition; (-) no bacterial growth. | ||||||
The results showed that all strains were susceptible to antibiotics (PC - disks of infusion). The results showed that the P. aeruginosa strain is as susceptible to BCM-A. vera extract as Penicillin – Streptomycin antibiotics disk. The S. aureus strain is susceptible to BCM-A. vera (loading rate of 225%). However, the delivery rate of A.vera extracts was not enough to inhibit the growth of E. coli strains. The delivery rate of C. officinalis extracts (loading rate of 225%) was not enough to inhibit the growth of P. aeruginosa and E. coli strains. The highest loading rate was for M. recutita (268%). However, the delivery rate did not inhibit the growth of any of the three bacterial strains.
These results are surprising from the water solubility point of view, as it was expected that the more water-soluble compound (hyperosides – C. officinallis) would have higher diffusion capacity and, consequently, more significant antibacterial activity. However, the best result was found for Aloe vera, whose polysaccharide has the potential for hydrogen-bonding interactions with cellulose [27]. Considering that all the strains used in this study were sensitive to extracts (Table 1), the findings showed that BCM did create a selective barrier for delivery, which did limit the antimicrobial activity of A. vera. C. officinalis and M. recutita.
Spectroscopy by FTIR
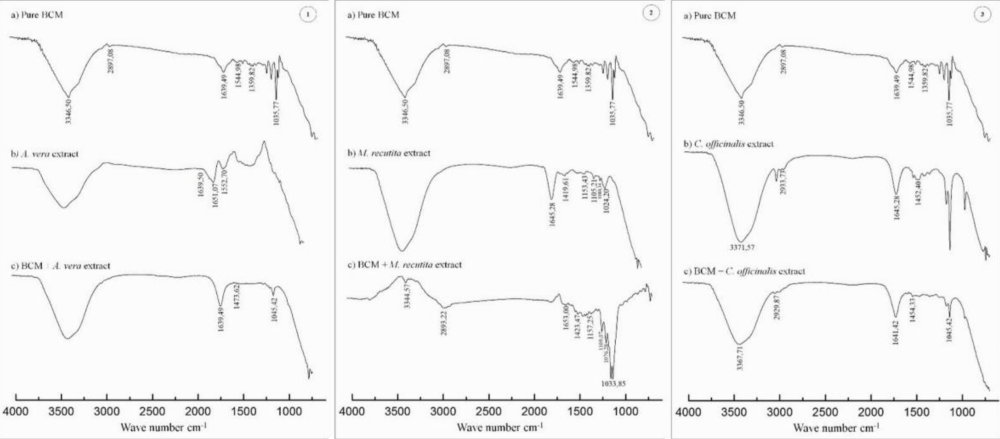
BCM spectrum band (Figure 1). is characterized by assigned to C−O−C stretching within an anhydroglucose ring in 1,035 cm-1, this band stretching can be found between 950 - 1,100 cm-1; C-H, C-H2, and C-H3 stretching between 1,320 and 1,466 cm-1; C-H and O-H stretching become visible, respectively, in 2,897 cm-1 and 3,346 cm-1 [28–31].
Figure 1: FTIR spectra of BCM pure and BCM - plant extract.
The Aloe vera transmittance band at 3,410 cm−1 was contributed by OH group of carbohydrate monomers including mannose and uronic acid [32]; bands at 1,639, 1493.83, 1244.27, 1060.69 and 876.93 cm−1 were of C-O stretching, asymmetrical and symmetrical COO stretching of carboxylate compounds, C-O-C stretching of COCH3 groups, C-O stretching of monosaccharide units in the branched regions such as galactose and glucan units, C-H group of carbohydrate monomers respectively [32] and finally bands at 772.72 and 621.90 cm−1 signified C-H bend of aromatic and alkynes [30]. The results of FTIR (Figure 1) for A. vera. C. officinalis and M. recutita and BCM are similar at finding in the literature [33]. In 1,544 cm-1 and 1,359 cm-1 bands are due NH2 group associated with axial deformation of the C = O group and primary and secondary amides [34–36].The axial deformation of secondary amides N-H in the BCM-A. vera are shown 1,473 cm-1, and they can be attribute hydrogen-bonding interactions between polysaccharides with cellulose [27]. The spectra (Figure 1) can help to explain the absence of activity of the M. recutita against all microbial strains. In the spectrum of the BCM – M. recutita the deformation O-H in 1639 cm-1 (BCM) and 1645 cm-1 (M. recutita) are suppressed. On the other hand, there is an increase in the absorption band in 1033 cm-1 and 1035 cm-1 of the BCM M. recutita spectrum. These results are an indication of bonding interactions of M. recutita polyphenols groups with the C-O deformation of bacterial cellulose membrane.
Differential scanning calorimetry
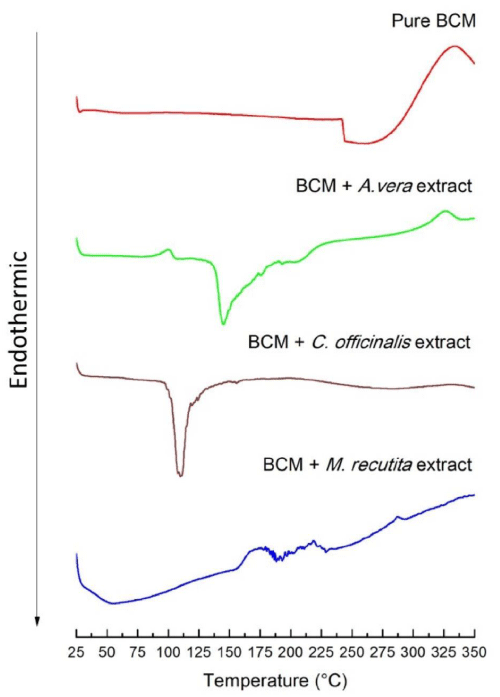
The thermograms curves by DSC are shown in Figure 2. The DSC curve for BCM show an abrupt endothermic transition event with onset at 240oC and end set near of 300oC, which was probably due to the decomposition of sulfates groups and the derivative compounds, in which salinization was formed during the bacterial culture process in the Hestrin & Schramm medium. The age of the plants and gel collection seasons influence the thermal properties of Aloe vera [37] as well as the procedures that involve dried using spray drying, freeze-drying, and refracting window drying methods [38]. The DSC thermogram curve of A. vera powder shows a sharp endothermic inflection at 406oC. The DSC thermogram curve of A. vera hydrated shows glass transition events between 27.53 ± 3.79oC with an enthalpy of 0.31 J. g-1.C-1 and 65.76 ± 0,09oC, with an enthalpy of 0.25 J. g-1.C-1 [38,39]. The results in the DSC curves in figure 2 (BCM- A. vera) shown degradation events in the temperatures of 90.2oC, which have a relation with monosaccharides of A. vera and 310oC due to BCM. Endothermic events, characteristics of melting point, in 148.5oC is due to disruption hydrogen-bonding interactions between polysaccharides - cellulose.
Figure 2: DSC curves of pure BCM, BCM - A. vera extract, BCM - C. Officinallis extract, and BCM - M. recutita extract.
Slavov, et al. 2016 showed a result of DSC curves for water-soluble pectic extracts from C. officinalis and M. chamomilla with an endothermic peaks maximum of 68,41oC and 74,61oC [40].
The results analyses figure 2 showed only one endothermic event in 115oC for C. officinalis. These results are similar to the results found Slavov, et al. [40] showing the peak maximum in 68.4oC [40]. Besides that, three exothermic events can be observed in 175.6oC and 290.2oC for M. recutita.
A general but safe analysis of DSC results is that extracts incorporated in BCM change the thermal properties of both the extract and BCM. However, in this work, the effects detected by DSC could not be the moment to be explained unambiguously. Other studies of FTIR and calorimetry analysis need to be done to understand the physical and chemical phenomena of the interaction between these extracts and BCM.
Mucoadhesive properties
The interface between the two different surfaces is assumed to act like a stretched membrane under tensile stress, which is a complementary measurement of mucoadhesion related to the balance of the cohesive forces acting at the interface at a molecular level.
Table 3 shows the results of the evaluation of mechanical properties as force detaches (N. s-1) i.e., mucoadhesion, work of adhesion (J.m-2), which is a measure of the strength of the contact between mucin disks and BCM. Tensile stress is the resultant between equal and opposite forces applied on the BCM surface, and it is required to hold the membrane in a position.
| Table 3: In vitro mechanical properties (M ± SD) for nature BCM and BCM-extracts. For all variables with the same letter, the difference between the average is not statistically significant (p > 0.05). Different letters are statistically significant (p < 0.05) | |||
| Samples | Force detaches N. sec | Work of adhesion J.m-2 |
Tensile stress N.cm-2 |
| Nature BCM | 0.47 ± 0.1a,c | 0.043 ± 0.005a | 0,93 ± 0.11a |
| BCM-A. vera | 0.24 ± 0.03b | 0.024 ± 0.005b | 0,47 ± 0.08b |
| BCM-C. officinalis | 0.57 ± 0.09a | 0.041 ± 0.01a | 7.12 ± 0.14c |
| BCM- M. recutita | 0.23 ± 0.17b,c | NA | NA |
| NA: Not Applicable. | |||
Although the averages show differences between the samples, statistically, the difference (p < 0.05) exists between native BCM compared with BCM-A. vera and BCM-M. recutita. The in vitro adhesive capacity of the samples measured on the mucin disc surface was determined by the force required to detach one interface from another.
The analysis of the results presented in table 3 show that the A. vera p < (0.05) and M. recutita (p > 0.05) decrease the mucoadhesive strength of BCM, while C. officinalis increases (p > 0.05). The work of adhesion it was that necessary must be done to separate the two adjacent phases. Conversely, it is the energy that is released in the process of detachment i.e., it is a measure of the strength of the contact between nature BCM or BCM-extract with the mucin. The work of adhesion of BCM-A. vera (p < 0.05) is lower than the nature BCM and BCM-M. recutita (p < 0.05). The work adhesion and tensile stress measures for M. recutita it is not conclusive due to substantial standard deviation. This result can be associated with of bonding interactions of M. recutita polyphenols groups with the C-O deformation of bacterial cellulose membrane (Figure 2), increasing anti plasticizing effect. BCM-C. officinalis is the most brittle material amongst all BCM thus showed highest tensile stress.
Biosynthesis of bacterial celluloses is controlled by multi-stage reactions involving complex catalytic regulatory proteins and several specific enzymes. In general, bacterial celluloses are a source of pure and crystalline cellulose. In the form of closely together nanofibers, it forms membranes that have been explored in topical drug delivery systems. However, the results presented in the literature are controversial, and reproducibility depends on the technique used in production. Due to this, bacterial cellulose as a carrier for drug delivery systems had shown controversial results and of hard comparison. In the other hand, the therapeutic activity of plant extracts also is involved by inherent peculiarities like plant origin, the season of harvest, type of extract (liquid or dry), extraction, and drying processes [41].
The execution of this work research was a challenge due to the standardization and quality of the BCM and vegetal extract. The minimum inhibitory concentration of antimicrobial activity of the A. vera, M. recutita, and C. officinalis extracts have corresponded to those described in the literature. However, when the extracts were incorporated at BCM, the antimicrobial activity of extracts changed in an importantly. The BCM-A. vera had not shown exhibited antimicrobial activity against E. coli. The BCM-C. officinalis had antimicrobial activity only against S. aureus, and the M. recutita had no activity against the microorganisms used in the test. FTIR spectra and DSC curve demonstrated the different types of interaction between BCM and each extract, and in vitro mucoadhesion properties. The important property of mucoadhesion on mucin disc suggests that BCM can be used to therapy of infected and ulcerated skin wounds, burns, and for mucosal tissues of the buccal and vaginal cavities. At least, we concluded that the bacterial cellulose membrane, despite its degree of purity, interacted chemically with actives compounds of the plant’s extracts, whether inhibiting or decreasing the biological activities. Meanwhile, for each specific situation, a detailed and careful study biopharmacotechnics is needed to be done, and all details of production need to be related.
São Paulo Research Foundation (FAPESP, São Paulo, Brazil) 2016/05930-4. Coordination for the Improvement of Higher Education Personnel (CAPES/ CNPq Brazil) for granting a scholarship. Group Center Flora. Biotae Vegetables Extracts.
Compliance with ethical standards
This article does not contain studies with human or animal participants by any of the authors. The manuscript is original and has not been submitted to any other journal. All authors read and approved the manuscript content. All authors contributed to the study.
- World Health Organization (WHO). Burns.
- Duke JM, Randall SM, Wood FM, Boyd JH, Fear MW. Burns and long-term infectious disease morbidity: A population-based study. Burns. 2017; 43: 273–281. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28041752
- Hassen AF, Khalifa S Ben, Daiki M. Epidemiological and bacteriological profiles in children with burns. Burns.2014; 40: 1040–1045. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24331406
- BraziL. Anvisa (National Health Surveillance Agency). Normative Instruction No. 5, of December 11, 2008. List of phytotherapeutic drugs of simplified registration. Ministry of Health. BraziL.
- BraziL. Anvisa (National Health Surveillance Agency). Normative Instruction No. 2, of May 13, 2014. List of phytotherapeutic drugs of simplified registration. Ministry of Health. BraziL.
- Martins MD, Marques MM, Bussadori SK, Martins MAT, Pavesi VCS, et al. Comparative Analysis between Chamomilla recutita and Corticosteroids on Wound Healing. An in vitro and In Vivo Study. Phyther Res. 2009; 23: 274-278. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18803230
- Hu JJ, Cui T, Rodriguez-Gil JL, Allen GO, Li J, et al. Complementary and alternative medicine in reducing radiation-induced skin toxicity. Radiat Environ Biophys. 2014; 53: 621–626. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24792319
- Schneider F, Danski MTR, Vayego SA. Usage of calendula officinalis in the prevention and treatment of radiodermatitis: A randomized double-blind controlled clinical triaL. Rev Esc Enferm USP. 2015; 49: 221–228. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25992820
- Liu TL, Miao JC, Sheng WH, Xie YF, Huang Q, et al. Cytocompatibility of regenerated silk fibroin film: A medical biomaterial applicable to wound healing. J Zhejiang Univ Sci B. 2010; 11: 10–16. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20043346
- Donini ÍAN, De Salvi DTB, Fukumoto FK, Lustri WR, Barud HS, et al. Biosynthesis and recent advances in bacterial cellulose production. Eclect Chem. 2010; 35: 165–178.
- Mogoşanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014; 463: 127–136. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24368109
- Jozala AF, de Lencastre-Novaes LC, Lopes AM, de Carvalho Santos-Ebinuma V, Mazzola PG, et al. Bacterial nanocellulose production and application: a 10-year overview. Appl Microbiol BiotechnoL. 2016; 100: 2063–2072. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26743657
- Rios AC, Moutinho CG, Pinto FC, Del Fiol FS, Jozala A, et al. Alternatives to overcoming bacterial resistances: state-of-the-art. Microbiol Res. 2016; 191: 51-80. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27524653
- Rahman H, Chandra A. Microbiologic Evaluation of Matricaria and Chlorhexidine against E. faecalis and C. albicans. Indian J Dent. 2015; 6: 60-64. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26097333
- Panghal M, Kaushal V, Yadav JP. in vitro antimicrobial activity of ten medicinal plants against clinical isolates of oral cancer cases. Ann Clin Microbiol Antimicrob. 2011; 10: 21. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21599889
- George D, Bhat SS, Antony B. Comparative evaluation of the antimicrobial efficacy of Aloe vera tooth gel and two popular commercial toothpastes: An in vitro study. Gen Dent. 2009; 57: 238-241. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19819812
- Michelin DC, Moreschi PE, Lima AC, Nascimento GGF, Paganelli MO, et al. Evaluation of the antimicrobial activity of vegetal extracts. Brazilian J Pharmacogn. 2005; 15: 316-320.
- Abudunia AM, Marmouzi I, Faouzi ME, Ramli Y, Taoufik J, et al. Anticandidal, antibacterial, cytotoxic and antioxidant activities of Calendula arvensis flowers. J Mycol Med. 2017; 27: 90–97. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28011127
- Cavalieri SJ, Rankin ID, Harbeck RJ, Sautter RL, McCarter YS, et al. Manual of antimicrobial susceptibility testing. Coyle MB, editor. Seatle, Washington: American Society for Microbiology. 2005; 242.
- NCCLS. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard-Eighth Edition. NCCLS document M2-A8. VoL. 23 No 1. Wayne, Pennsylvania, USA; 2003.
- Jozala AF, Pértile RA, dos Santos CA, de Carvalho Santos-Ebinuma V, Seckler MM, et al. Bacterial cellulose production by Gluconacetobacter xylinus by employing alternative culture media. Appl Microbiol BiotechnoL. 2014; 99: 1181-1190. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25472434
- Shezad O, Khan S, Khan T, Park JK. Physicochemical and mechanical characterization of bacterial cellulose produced with an excellent productivity in static conditions using a simple fed-batch cultivation strategy. Carbohydr Polym. 2010; 82: 173–180.
- Ferro VA, Bradbury F, Cameron P, Shakir E, Rahman SR, et al. in vitro susceptibilities of Shigella flexneri and Streptococcus pyogenes to inner gel of Aloe barbadensis Miller. Antimicrob Agents Chemother. 2003; 47: 1137–1139. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12604556
- Arunkumar S, Muthuselvam M. Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J Agric Sci. 2009; 5: 572–576.
- Kazemi M. Chemical Composition and Antimicrobial Activity of Essential Oil of Matricaria recutita. Int J Food Prop. 2015; 18: 1784-1792.
- Saibuatong O ard, Phisalaphong M. Novo Aloe vera-bacterial cellulose composite film from biosynthesis. Carbohydr Polym. 2010; 79: 455–460.
- Brunner G. Hydrothermal and Supercritical Water Processes. VoL. 5. Kiran E, editor. Amsterdam. 2014; 666.
- Sekiguchi Y, Sawatari C, Kondo T. A gelation mechanism depending on hydrogen bond formation in regioselectively substituted O-methylcelluloses. Carbohydr Polym. 2003; 53: 145–153.
- Oliveira RL, Vieira JG, Barud HS, Assunção RMN, Filho GR, et al. Synthesis and characterization of methylcellulose produced from bacterial cellulose under heterogeneous condition. J Braz Chem Soc. 2015; 26: 1861–1870.
- Lim ZX, Cheong KY. Effects of drying temperature and ethanol concentration on bipolar switching characteristics of natural Aloe vera-based memory devices. Phys Chem Chem Phys. 2015; 17: 26833–26853. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26400096
- Balaji A, Jaganathan SK, Supriyanto E, Muhamad II, Khudzari AZM. Microwave-assisted fibrous decoration of mPE surface utilizing Aloe vera extract for tissue engineering applications. Int J Nanomedicine. 2015; 10: 5909–5923. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26425089
- Dutta D, Mukherjee R, Patra M, Banik M, Dasgupta R, et aL. Green synthesized cerium oxide nanoparticle: A prospective drug against oxidative harm. Colloids Surfaces B Biointerfaces. 2016; 147: 45–53. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27478962
- Barud HS, Souza JL, Santos DB, Crespi MS, Ribeiro CA, et al. Bacterial cellulose/poly(3-hydroxybutyrate) composite membranes. Carbohydr Polym. 2011; 83: 1279–1284.
- Déléris G, Petibois C. Applications of FT-IR spectrometry to plasma contents analysis and monitoring. Vib Spectrosc. 2003; 32: 129–136.
- Lin WC, Lien CC, Yeh HJ, Yu CM, Hsu SH. Bacterial cellulose and bacterial cellulosechitosan membranes for wound dressing applications. Carbohydr Polym. 2013; 94: 603– 611. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23544580
- Godinho JF. Bacterial Cellulose Hydrogels Incorporated with Aloe vera Fractions. Florianólis: Federal University of Santa Catarina (UFSC); 2014.
- Ray A, Ghosh S, Ray A, Aswatha SM. An analysis of the influence of growth periods on potential functional and biochemical properties and thermal analysis of freeze-dried Aloe vera L. geL. Ind Crops Prod. 2015; 76: 298–305.
- Raghavi LM, Moses JA, Anandharamakrishnan C. Refractance window drying of foods: A review. J Food Eng. 2018; 222: 267–275.
- Aghamohamadi N, Sanjani NS, Majidi RF, Nasrollahi SA. Preparation and characterization of Aloe vera acetate and electrospinning fibers as promising antibacterial properties materials. Mater Sci Eng C Mater Biol AppL. 2019; 94: 445–452. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30423728
- Slavov A, Panchev I, Kovacheva D, Vasileva I. Physico-chemical characterization of water-soluble pectic extracts from Rosa damascena, Calendula officinalis and Matricaria chamomilla wastes. Food HydrocolL. 2016; 61: 469–476.
- World Health Organization (WHO). Annex 1 WHO guidelines on good herbal processing practices for herbal medicines. WHO Tech Rep Ser No 1010. 2018; 83–149.