More Information
Submitted: March 21, 2022 | Approved: March 30, 2022 | Published: March 31, 2022
How to cite this article: Simonyi M. Revisiting carotenoid aggregates discerning non-covalent interaction of unbranched fatty acid analogues. Arch Pharm Pharma Sci. 2022; 6: 001-005.
DOI: 10.29328/journal.apps.1001028
Copyright License: © 2022 Simonyi M. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Exciton coupling; Dihedral angle; Types of aggregates; Overlay angle; Lipid additives
Revisiting carotenoid aggregates discerning non-covalent interaction of unbranched fatty acid analogues
Miklós Simonyi*
Research Center for Natural Sciences, Institute of Organic Chemistry, Budapest, Hungary
*Address for Correspondence: Miklós Simonyi, Research Center for Natural Sciences, Institute of Organic Chemistry, Budapest, Hungary, Email: [email protected]
Certain carotenoid aggregates provide scaffolds of moderate stability characterized by their CD spectra. The interaction of the scaffold with small lipid molecules either destabilize, or reinforce its structure. The idea can be applied to open-chain fatty acids and some analogues. Fatty acids and fatty alcohol decrease exciton intensity of the aggregate, while esters of both alcohol and acids increase it. Moreover, the stabilizing effect depends on chain-length. Thus, the scaffold distinguishes compounds that either loosen its structure, or reinforce it.
Experimental investigations on carotenoid aggregates intensified in the beginning of this century. Observations were mainly due to exciton phenomena the theoretical background of which have been developed for covalently bonded chromophors [1,2]. Several kinds of aggregates were observed and characterized. However – beyond early phenomenological description – no idea was put forward about their use for any purpose. This paper describes a potential application of the aggregate.
The exciton phenomenon
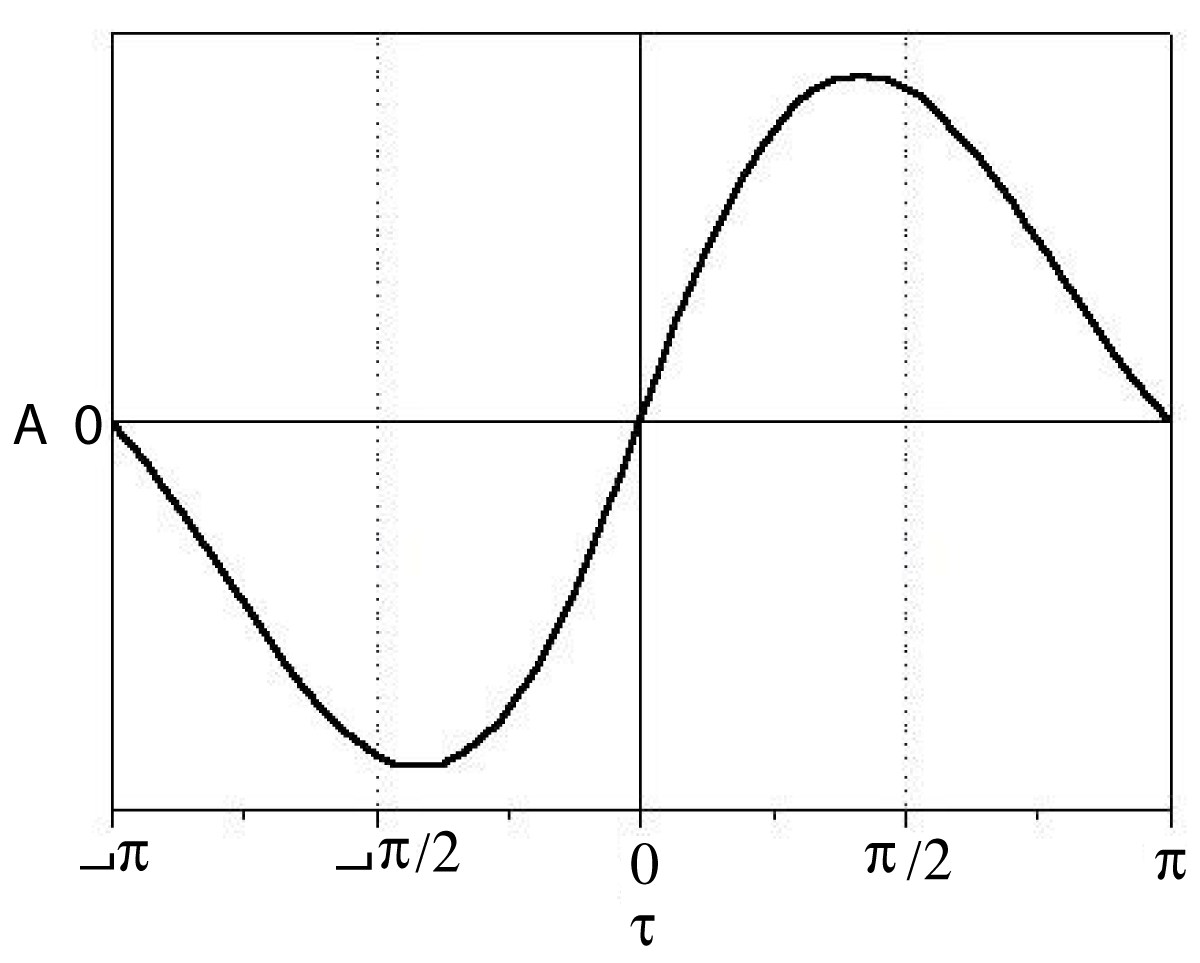
Molecular chromophores become excited upon irradiation that is experimentally detectable by spectroscopy. Absorption of light transfers energy to the electrons of the chromophore, predominantly to those involved in conjugated double bonds, or aromatic rings. The absorbed energy moves the electrons to higher-energy molecular orbital. It is denoted by π → π* excitation creating an electric transition moment which is a vector quantity having both a direction and a magnitude (intensity) characteristic of the chromophore involved. The direction of electric transition vector lies alongside the conjugated π-electron systems. When two chromophores are in vicinity to each other and one of them became excited, the other may become excited too owing to their interaction through space, even if their π-electrons are separated. This delocalized excitation – called exciton – has spectral consequences; the UV-vis absorption band splits (exciton splitting) into two, red-shifted and blue-shifted ones that sometimes appear by broadening the original band. If the interacting chromophores are localized in chiral array, the exciton can be detected by the circular dichroism (CD) spectrum that plots differential absorption [Δε] against wavelength demonstrating separated positive and negative bands. The magnitude of exciton is defined by the CD spectrum as A = Δε1 – Δε2 depending on both the nature of chromophores and the dihedral angle [τ] of their electric transition moments. As Harada demonstrated [3], the A value does not change sign for torsion angles falling within the interval of 0° < τ < 180° and passes through a maximum around 70°. It is true for 180° < τ < 360° – the other hemicycle – too, with opposite signs for the CD bands.
Figure 1 illustrates the dependence for the full cycle.
Figure 1: The completed Harada curve for right-handed exciton intensity of identical chromophores.
Dihedral angle (δ)
The connection between spectra and chemical structure is strong; it was shown by Harada and Nakanishi [3] how CD spectra could be used for the determination of absolute configuration.
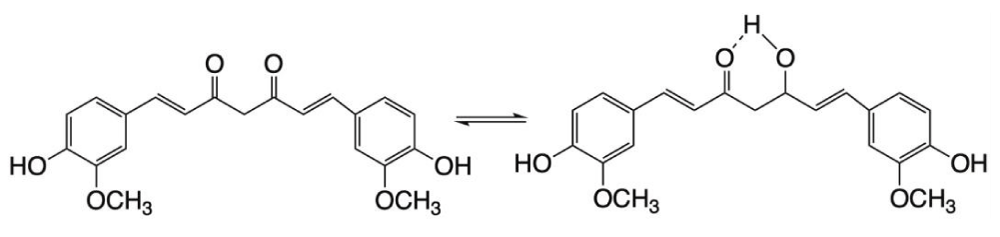
The relation of exciton spectra to structure can be demonstrated by curcumin [4] (Figure 2).
Figure 2: Keto-enol tautomers of curcumin.
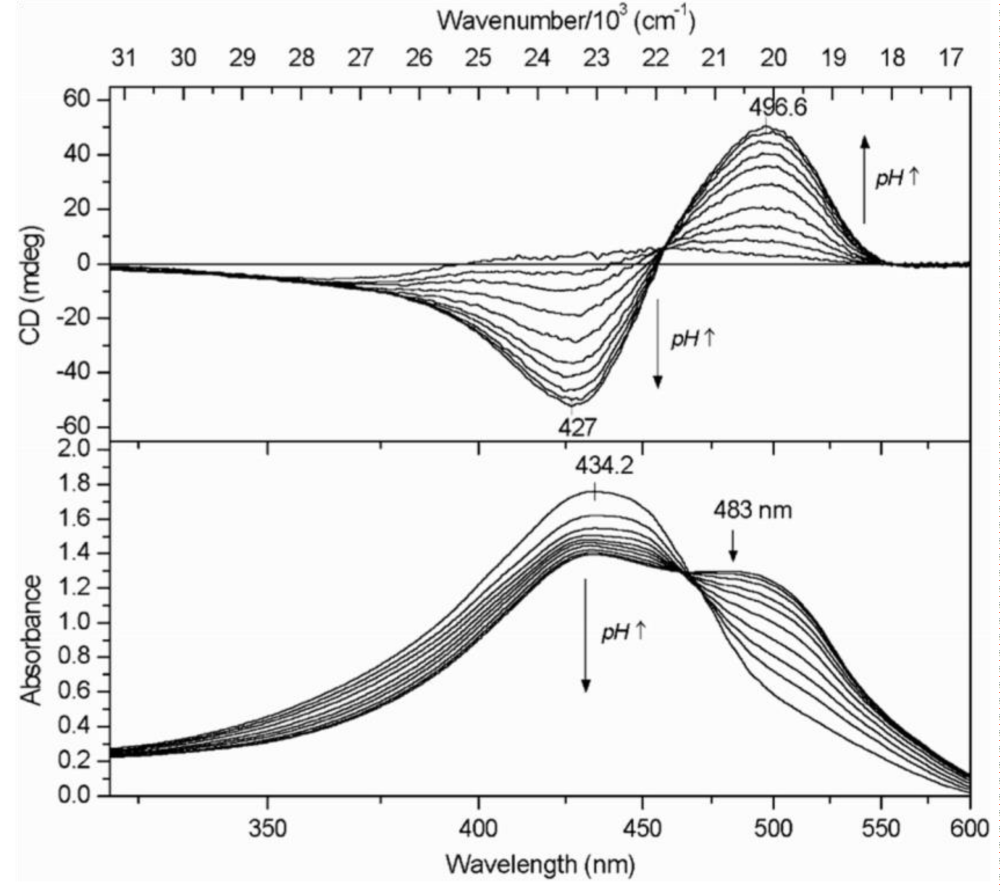
This di-phenolic molecule exists in the form of two tautomers. The keto form has two symmetrical isolated chromophores. Both isolation and symmetry vanish in the enol form containing conjugated double bonds of the whole molecule. Curcumin has UV-vis spectra, but no CD owing to its flexibility that cancels out the deviation of its conformations from planarity. But the molecule may become stiff when it is bound to a surface, e. g. to human serum albumin (HSA). At neutral pH the conformation of bound curcumin remains essentially planar, thus preventing its two half-parts to produce exciton coupling. However, the phenolic groups become charged in alkalic media allowing additional interactions of curcumin with the surrounding amino acids which forces the molecule out of plane. These changes are seen on the UV-vis and CD spectra (Figure 3).
Figure 3: The dependence of UV-vis and CD spectra on pH for curcumin-HSA complex. (Taken from ref. 4, by courtesy of Elsevier).
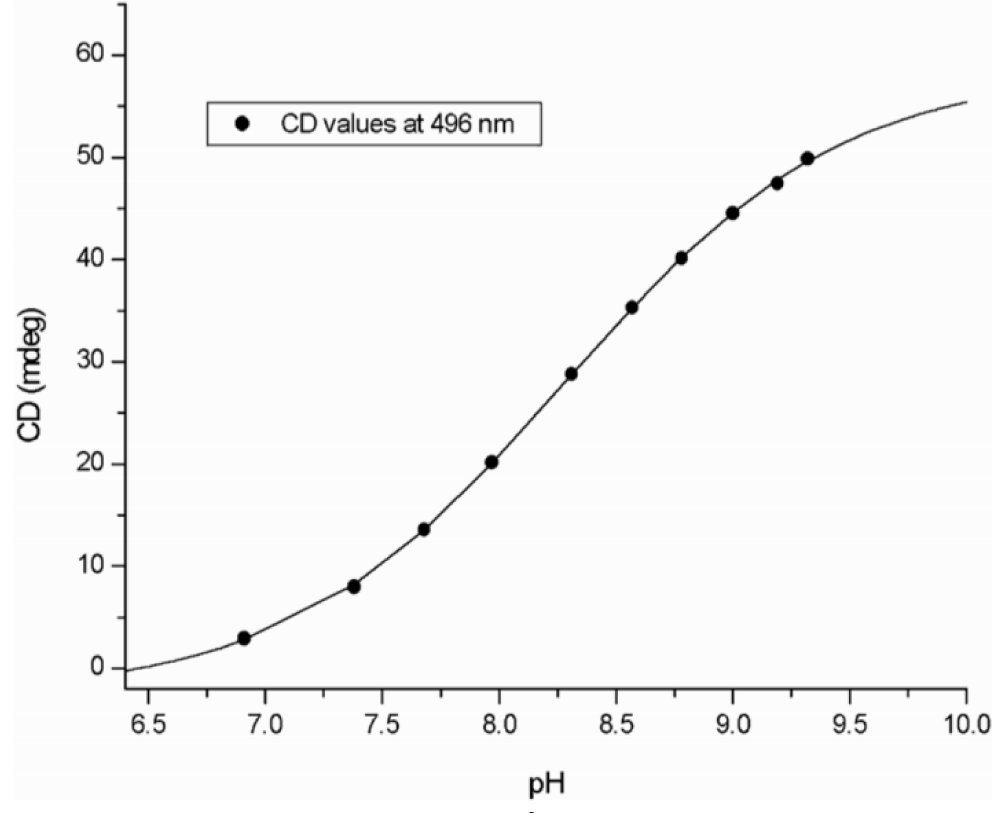
With increasing pH right-handed exciton spectra of curcumin were obtained testifying a gradual conformational torsion of the molecule. The dependence is more clearly seen by plotting the intensities of the induced positive CD band against pH (Figure 4).
Figure 4: The magnitude of the long wavelength CD band of curcumin-HSA complex depends on pH. (Taken from ref. 4, by courtesy of Elsevier).
Since changing pH has no effect on the spectral contribution of HSA, these spectra indicate a gradual increase of the dihedral angle between the half-curcumin chromophores. A molecular model characterizes the conformation curcumin adopts at high pH (Figure 5).
Figure 5: Model of curcumin bound to HSA at high pH, δ = +156°. (Taken from ref. 4, by courtesy of Elsevier).
Development of aggregates
Carotenoid samples were prepared in water-miscible organic solvents at sub-millimolar concentration [5-9]. After aqueous dilution (3 times v/v), all samples remained clear, suitable for spectrometric analyses with no sign of opalescence or precipitation.
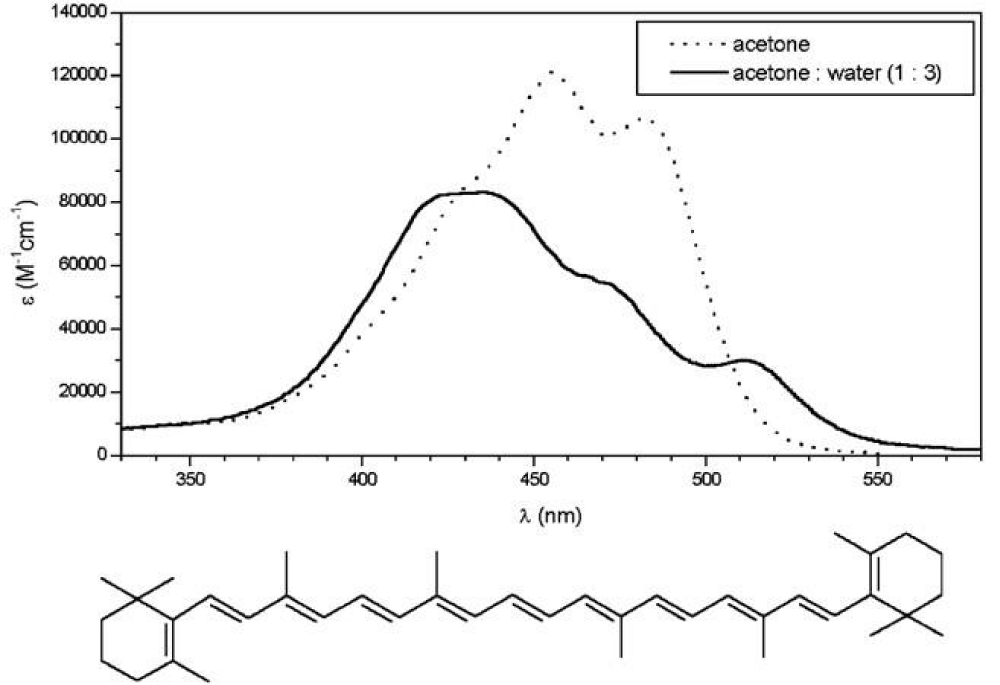
The principal difference of covalently linked chromophores from aggregates (subject to self-assembly) is that – instead of dihedral angles (δ) – we are dealing with the overlay angle (ω) of neighboring molecules. For carotenoid aggregates, the individual molecules can roughly be regarded having long linear chromophores (conjugated double bonds) that form the aggregate as overlaying sticks. How these can assemble depends heavily on their structure. The simplest carotenoids are hydrocarbons without oxygen functions. These form aggregates like haystacks lacking definite structure [5], as shown in Figure 6.
Figure 6: The UV-vis spectra of β-carotene. (Taken from ref. 5, by courtesy of Elsevier).
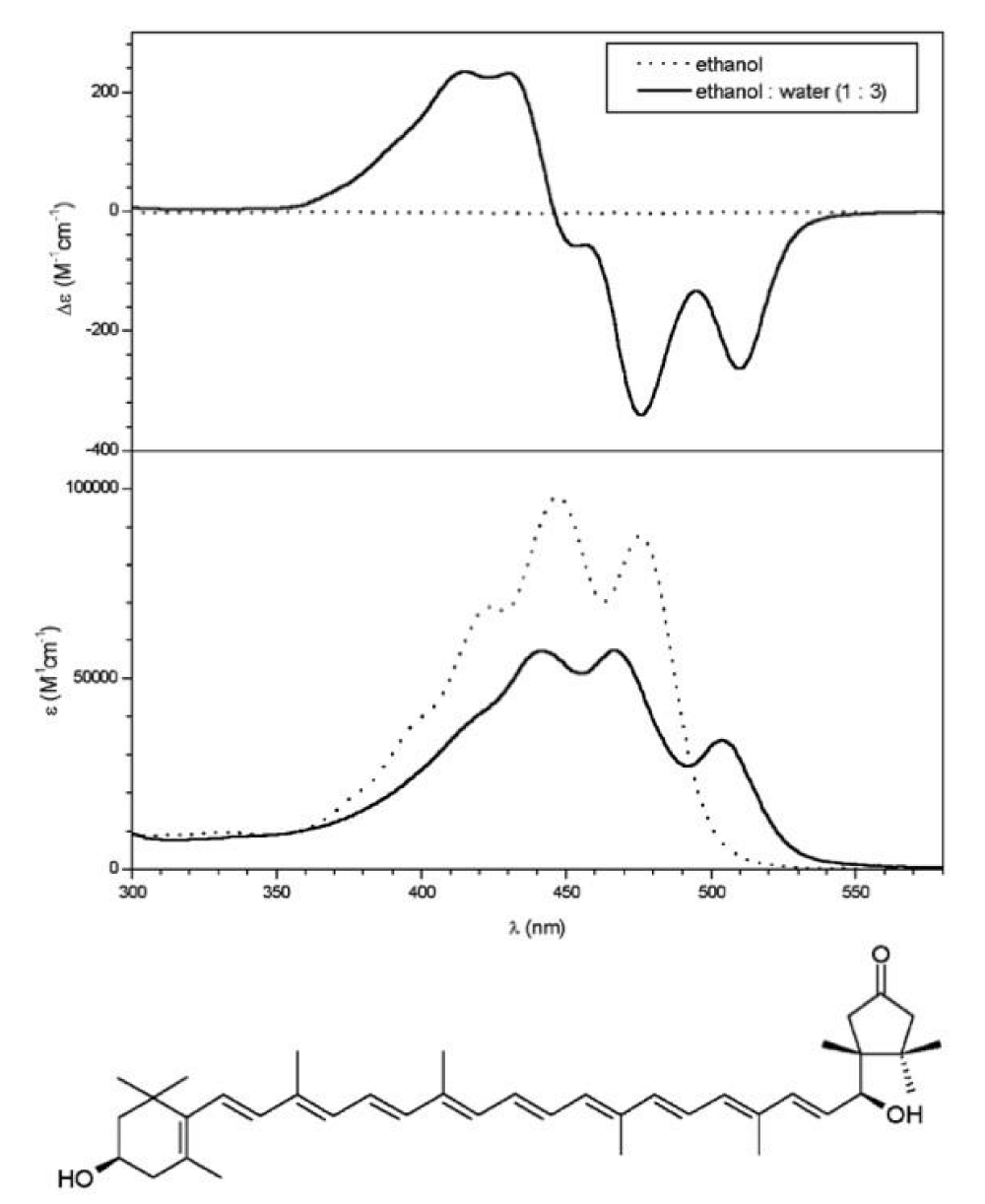
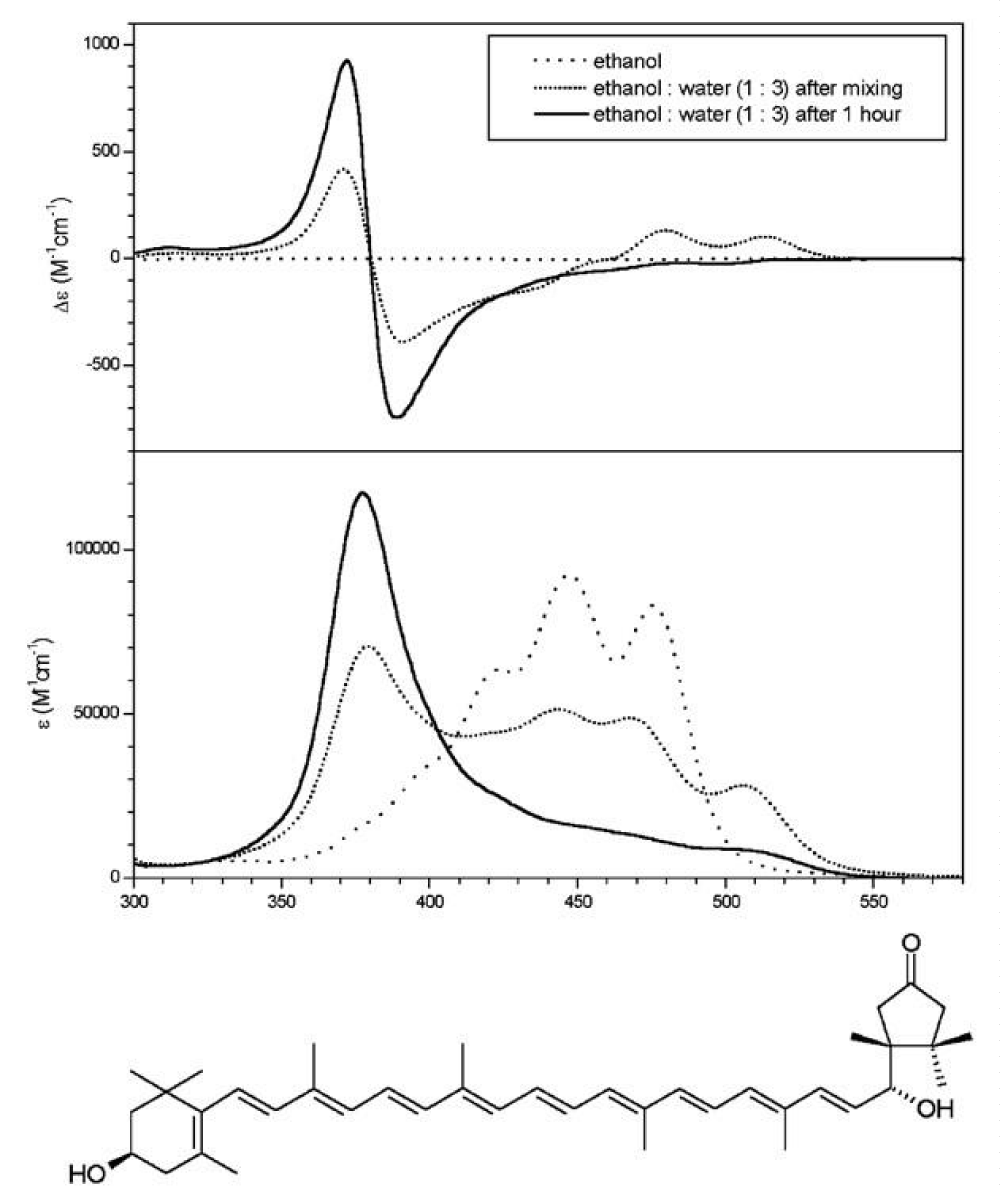
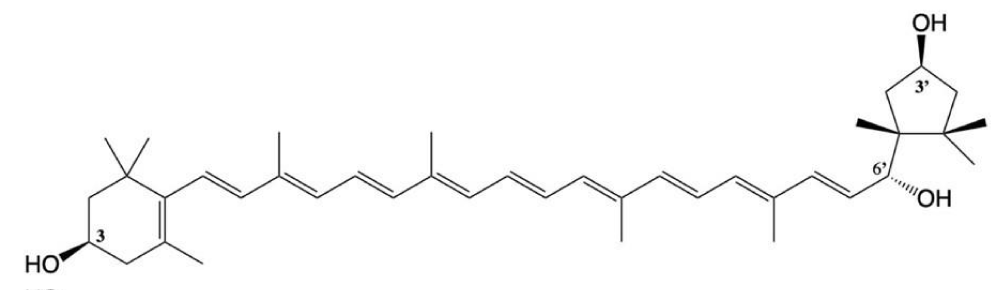
But when the carotenoid has hydroxyl groups at both ends of the molecule, CD spectra indicate oriented molecules in the aggregate. It appeared immediately, that chirality in the 6’position has a very important role in the aggregate structure. While the broad CD-band of 3S,6’S-capsanthol-3’-on displaying several extrema indicates a variety of structured aggregates (Figure 7), the spectra of its epimer, 3S,6’R-capsanthol-3’-on (Figure 8) show a highly restricted, tight arrangement according to its sharp, highly intensive CD-peaks [5]. Longwavelength CD-bands of both epimers are negatve (left-handed aggregates).
Figure 7: Spectra of 6’S-capsanthol-3’-on. (Figures taken from ref. 5, by courtesy of Elsevier).
Figure 8: Spectra of 6’R-capsanthol-3’-on. (Figures taken from ref. 5, by courtesy of Elsevier).
For the systematic investigation on the role of endgroups in the aggregate structure [6,7], we selected (3S,3’S,6’R)-capsanthol (Figure 9). This cartenoid gives very similar aggregate to that of 3R,6’S-capsanthol-3’-on. Partial acetylation of the three hydroxyl groups [7] provided models demonstrating that tightly packed aggregate formed only if the configuration at 6’ was R and free hydroxyl groups were present at both ends.
Figure 9: (3S,3’S,6’R)-capsanthol.
Characterizing the structure of tight aggregates to be in conform with spectral properties, one has to assume they should be closely packed forming a left-handed array. We selected 3S,6’R-capsanthol-3’on (Figure 8) for illustration. In order to overcome steric hindrance of bulky end groups, the essential hydroxyl groups must orient close overlay of neighboring molecules in the aggregate [8]. Besides, neighboring dimers in the aggregate should display the lowest interface with the aqueous solvent. Based on these considerations we suggested (1) optimal overlay angle to be ω = – 20° and (2) a continuous chain of hydrogen bonds linking neighboring carotenoid molecules to each other in the following fashion:
(6’ – OH)first molecule →(3’ – O=C)second molecule
(3’ – O=C)second molecule →(6’ – O–H)third molecule
and so on, while the other end of molecules have weaker interactions between identical (3S – OH) groups. Accordingly, the tight aggregate assumes helical arrangement in which 18 carotenoid chains complete a full cycle (18 x 20° = 360°). Based on these considerations, the structure of tightly packed aggregate may be envisaged, as shown in Figures 9.
Testing the structure of tightly packed aggregate
Robustness of the tightly packed aggregate was demonstrated by the simultaneous development of aggregates from a mixture of 6’R-capsanthol and 6’S-capsanthol. Instead of decreasing, the intensity slowly increased, as if the tightly packed aggregate would serve as template for 6’S-capsanthol the latter being unable by itself to produce exciton of high intensity [9] (Figures 10).
Figure 10: Tightly packed aggregate of (3S,6’R)-capsanthol-3’-on. (Taken from ref. 8, by courtesy of Wiley).
At that point it was tempting to investigate the development of aggregates in the presence of lipid additives lacking any chromophore to see their influence on the tightly packed aggregate. The lipid character in aqueous environment ensures close interaction with the carotenoid chains. The following fatty acids, alcohol and their derivatives have been selected:
| Dodecanol | C12H25–OH | Dodecanol acetate | C12H25–OCOCH3 |
| Lauric acid | C11H23–COOH | Ethyl laurate | C11H23–COOC2H5 |
| Tridecanoic acid | C12H25–COOH | ||
| Myristic acid | C13H27–COOH | Ethyl myristate | C13H27–COOC2H5 |
| Palmitic acid | C15H31–COOH | Ethyl palmitate | C15H31–COOC2H5 |
| Table 1: Variation of exciton intensity (A0) brought about by additives (Ai) | |||
| Additive | Exciton intensity | α | |
| Non | – 2500 | 1,00 | |
| Dodecanol | (C-12) | – 875 | 0,35 |
| Dodecanol acetate | (C-12) | – 2718 | 1,09 |
| Lauríc acid | (C-12) | – 719 | 0,29 |
| Tridecanoic acid | (C-13) | – 1143 | 0,46 |
| Myristic acid | (C-14) | – 896 | 0,36 |
| Palmitic acid | (C-16) | – 642 | 0,26 |
| Ethyl laurate | (C-12) | – 3618 | 1,45 |
| Ethyl myristate | (C-14) | – 4634 | 1,85 |
| Ethyl palmitate | (C-16) | – 4894 | 1,96 |
All additives containing –OH, or –COOH groups reduce the exciton intensity. These effects seem to be independent of chain length. They may be accounted for by their competitive interaction with those hydrogen bonds that hold individual carotenoid molecules of the aggregate together.
In contrast, all esters increased exciton intensity with fatty acid esters being more efficient than the alcohol acetate. Since the additives are not chromophores and unable to absorb light, they cannot contribute to exciton induction by their own. Thus, their effect can only be a modification of aggregate structure in a way which increases the A value. As we saw in the case of curcumin, exciton coupling increased along with widening dihedral angle, a parameter we lack in the aggregate. Instead, the overlay angle of neighboring carotenoid chains plays identical role. Its increase would happen by intercalation of the ester between carotenoid chains stretching them somewhat apart. In this context noteworthy is the trend the influence of esters display; α values increase with increasing chain length in the C-12 – C-16 interval.
Carotenoids contain 18 carbon atoms between cyclic end groups and shorter fatty acid chains could provide smaller strain for widening the overlay angle.
The results have the following consequences:
Practically, they are in accord with the structure of tightly packed aggregate. Theoretically, they prove the applicabilty of the completed Harada curve to interpret excitons of non-covalently linked chromophores.
Experimentally, the tightly packed aggregate can be used to test interactions with lipid compounds lacking chromophors. These interactions can still be detected by modified exciton signals.
A special thank is due to Professor József Deli, University of Pécs, who provided a large variety of carotenoid samples. The author thanks for financial support from projects of the Hungarian Research Fund, nos. 1/047 NKFP Medichem, OTKA T030271, T049721, T33109, and to his co-authors listed in the reference list.
- Harada N, Nakanishi K. Circular Dichroic Spectroscopy – Exciton Coupling in Organic Stereochemistry. University Science Books, Mill Valley, CA, 1983.
- Berova N, Harada N, Nakanishi K. Electronic Spectroscopy: Exciton Coupling, Theory and Applications. In: Lindon, J, Tranter, G, Holmes, J. (eds). Encyclopedia of Spectroscopy and Spectrometry. Academic Press, New York, 2000; 470–488.
- Harada N, Chen SL, Nakanishi K. Quantitative definition of exciton chirality and the distant effect in the exciton chirality method. J Am Chem Soc. 1975; 97: 5345-5352.
- Zsila F, Bikádi Z, Simonyi M. Molecular basis of the Cotton effects induced by the binding of curcumin to human serum albumin. Tetrahedron Asymmetry. 2003; 14: 2433-2444.
- Zsila F, Bikádi Z, Deli J, Simonyi M. Configuration of a single centre determines chirality of supramolecular carotenoid self-assembly. Tetrahedron Lett. 2001; 42: 2561-2563.
- Zsila F, Deli J, Bikádi Z, Simonyi M. Supramolecular assemblies of carotenoids. Chirality, 2001, 13, 739-744. PubMed: https://pubmed.ncbi.nlm.nih.gov/11746813/
- Bikádi Z, Zsila F, Deli J, Mády GY, Simonyi M. The supramolecular structure of self-assembly formed by capsanthin derivatives. Enantiomer, 2002; 7: 67-76. PubMed: https://pubmed.ncbi.nlm.nih.gov/12108636/
- Simonyi M, Bikádi Z, Zsila F, Deli J. Supramolecular exciton chirality of carotenoid aggregates. Chirality. 2003; 15: 680-698. PubMed: https://pubmed.ncbi.nlm.nih.gov/12923806/
- Zsila F, Bikádi Z, Deli J, Simonyi M. Chiral detection of carotenoid assemblies. Chirality. 2001; 13: 446-453.
- Simonyi M. Factors affecting supramolecular exciton intensity. Chirality. 2010; 22: E183-E185