More Information
Submitted: April 25, 2023 | Approved: May 08, 2023 | Published: May 09, 2023
How to cite this article: da Costa Vieira P, Cavalcanti LT, Dantas e Sousa Almeida HM, de Sousa Oliveira I, Ferreira SB. Cytotoxic Effects of Aminotriles with Bioactive Potential: An Integrative Review. Arch Pharm Pharma Sci. 2023; 7: 022-027.
DOI: 10.29328/journal.apps.1001040
Copyright License: © 2023 da Costa Vieira P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Aminonitriles; Cytotoxicity; Cellular effects; Drug development
Cytotoxic Effects of Aminotriles with Bioactive Potential: An Integrative Review
Paola da Costa Vieira1, Lettícia Tenório Cavalcanti2, Hélida Maravilha Dantas e Sousa Almeida3, Igor de Sousa Oliveira1 and Sávio Benvindo Ferreira4*
1Medical Student, Academic Unit of Life Sciences (UACV), Teacher Training Center (CFP), Federal University of Campina Grande (UFCG), 58900-000, Cajazeiras, Paraiba, Brazil
2Medical Student, Center for Biological and Health Sciences (CCBS), Federal University of Campina Grande (UFCG), 58429-600, Campina Grande, Paraíba, Brazil
3Bachelor of Science in Nursing, Postgraduate Program in Pharmaceutical Sciences, Health Sciences Center, Federal University of Rio Grande do Norte, Natal, Rio Grande do Norte
4PhD in Pharmacology, Professor of Microbiology, Academic Unit of Life Sciences (UACV), Teacher Training Center (CFP), Federal University of Campina Grande (UFCG), 58900-000, Cajazeiras, Paraíba, Brazil
*Address for Correspondence: Sávio Benvindo Ferreira, Microbiology Laboratory (CT-Infra), Teacher Training Center (CFP), Federal University of Campina Grande (UFCG), 58900-000, Cajazeiras, Paraíba, Brazil. Email: [email protected]
Aminonitriles are pharmacological-interest bioactive due to their promising antimicrobial and antitumor activity. Since cytotoxicity tests are inherent to the new drug development process, this work aimed to verify reports in the scientific literature on the cytotoxic effects of aminonitriles. The method adopted was an integrative review of works published in the last 10 years in the PubMed, Embase, Web of Science, and Virtual Health Library (VHL) databases. Three articles that matched the selection and eligibility criteria were included in this review. A total of 33 aminonitriles were used in the cytotoxicity experiments, and of the nine molecules based on pyridine, two exerted moderate cytotoxic activity, of the twelve synthesized from benzimidazole, none showed cytotoxic activity, and of the twelve derived from renieramycins, all showed considerable cytotoxic activities. The studies used in this research evaluated the cytotoxic effects of aminonitriles with evident anticancer and antimicrobial activity. The importance of evaluating the cytotoxicity of aminonitriles is emphasized, as well as the need for investigative research that explores other evaluation methods in pre-clinical tests that may corroborate the existing findings, with a view to the development of therapies against emerging health problems.
Aminonitriles are biosynthetic natural products, which have a profound impact on the biomedical sciences. This fact is justified, since their production is cheap, in addition to being molecules of vital importance for pharmacology, as they intermediate the chemical synthesis of complex alkaloids, preparation of new heterocycles, and drugs containing α-aminonitriles [1].
According to Grundke, Vierengel and Opatz [2], the use of bioactive compounds, such as aminonitriles, since they are hydrolyzed into amino acids, have a high reactivity and attractiveness. In addition, there is a hypothesis that these subunits, fruits of the Strecker reaction, showed a controlled ability to reach the cellular target since they have a strong potential to donate a grouping of their molecular arrangement, such as the carboxyl, ensuring that the compound receptor performs a better specific activity [3]. In addition, scientific literature progresses in bringing positive results regarding the use of aminonitriles against pathogenic microorganisms, such as Staphylococcus aureus and Mycobacterium tuberculosis and even antitumor activity [4]. In this sense, taking into account the diverse biochemical properties of aminonitriles, these substances represent a new possibility for the pharmaceutical industry.
In this perspective, cytotoxicity tests are necessary and comprise the initial stage of biocompatibility analysis, whose primary objective is to evaluate the safety of the molecule of interest and serve as an exclusion criterion [5]. Because of this, it is of fundamental importance to know the cellular effects of these substances, taking into account the fact that many promising molecules are discarded due to their ability to cause undesirable cellular damage and injuries [6].
Therefore, considering the potentially relevant biological activities of aminonitriles and emphasizing the need to develop alternative and/or complementary drugs that are safe for the treatment of pathologies of interest to public health, this study aims to analyze the evidence available in the literature on the cytotoxicity of this group of molecules.
Characterization of the study
The descriptive-exploratory method was adopted, with a qualitative approach, characterized by an integrative systematic review of the scientific literature. For that, the steps present in the research protocol by Dhollande, et al. [7], order to ensure greater methodological rigor to the investigation.
Problem identification
The research question was supported by the use of the acronym PICo, which represents: P - Population, I - Interest, and Co - Context - elements that direct the construction of the guiding question [8]. Therefore, the investigation was guided by the question: “What does the scientific literature point out about the cellular effects and the possible cytotoxicity of aminonitriles?”.
Literature search and presentation of search criteria
The bibliographic survey took place during the month of October 2022, directly in the databases: PubMed, Embase, Web of Science, and Virtual Health Library (VHL). For this, the search formula “(aminonitrile OR aminonitriles) AND (cytotoxic OR cytotoxicity)” was used, with the descriptors obtained from the Medical Subject Headings (MeSH). The selected works contemplated the following selection criteria: the complete articles available online, published in the last 10 years, in Portuguese, English, or Spanish, in this sequence. On the other hand, the exclusion criteria were: duplicate studies, review studies, and opinion articles, in addition to works that did not answer the guiding question of the research.
It should be noted that the described search period was selected due to the need to find relevant works regarding this class of bioactives, which, being recent, would not justify a longer period to find biological assays that explore the cytotoxic effects of these molecules.
Data characterization and evaluation
The eligibility stage occurred through the preliminary reading of titles and abstracts. Then, the works were read in full, and those that answered the guiding question of the study were included for data collection. All these processes were carried out by two researchers, independently, and in case of an impasse, their resolution would be based on the opinion of a third researcher, blindly.
Subsequently, data were collected independently by different investigators, and, in the end, their results were compared. A previously made instrument was used to systematize this process. The extracted information referred to the journal (title, reference, place, and type of publication) and the study (type of study, evidence, objective, methodological aspects and results, and level of evidence of the study).
Data collection, evidence analysis and presentation of results
During the evidence analysis process, the categorization of Agency for Healthcare Research and Quality (AHRQ) was used, where study quality is classified into six levels, with meta-analysis works of multiple controlled studies being the highest level, and the lowest, papers with opinion from reputable authorities based on clinical competence or expert committee opinion, including interpretations of information not based on research [9]. Data analysis was based on the results of the careful evaluation of the selected articles, with a comparison being made with theoretical knowledge and identification of conclusions. The presentation of results followed the orientation presented in the PRISMA checklist [10,11].
Assessment of the risk of bias of the studies analyzed
Although systematic reviews are vulnerable to biases that can mask the true results of the study, a series of measures can be taken to mitigate this problem in the conduct of the study. According to Almeida and Goulart [12] the types of biases in literature reviews can be classified into the following categories: selection bias, information bias, and confounding bias. In this way, the present work sought to mitigate its risk by adopting the following strategies:
1. Adoption of a careful study selection methodology carried out by two investigators individually, PCV and LTC, without sharing data.
2. Complete reading of all included works and evaluation of the information selected to compose this study by two researchers, PCV and LTC.
3. In addition, the complete review of the work was carried out in collaboration with two other researchers, IOS and HMDSA, and under the guidance of a fifth SBF researcher in order to ensure the methodological rigor stipulated for this work.
Ethical aspects
This study did not need to be submitted to a Research Ethics Committee (REC) as it is an integrative literature review and used public domain sources for its performance.
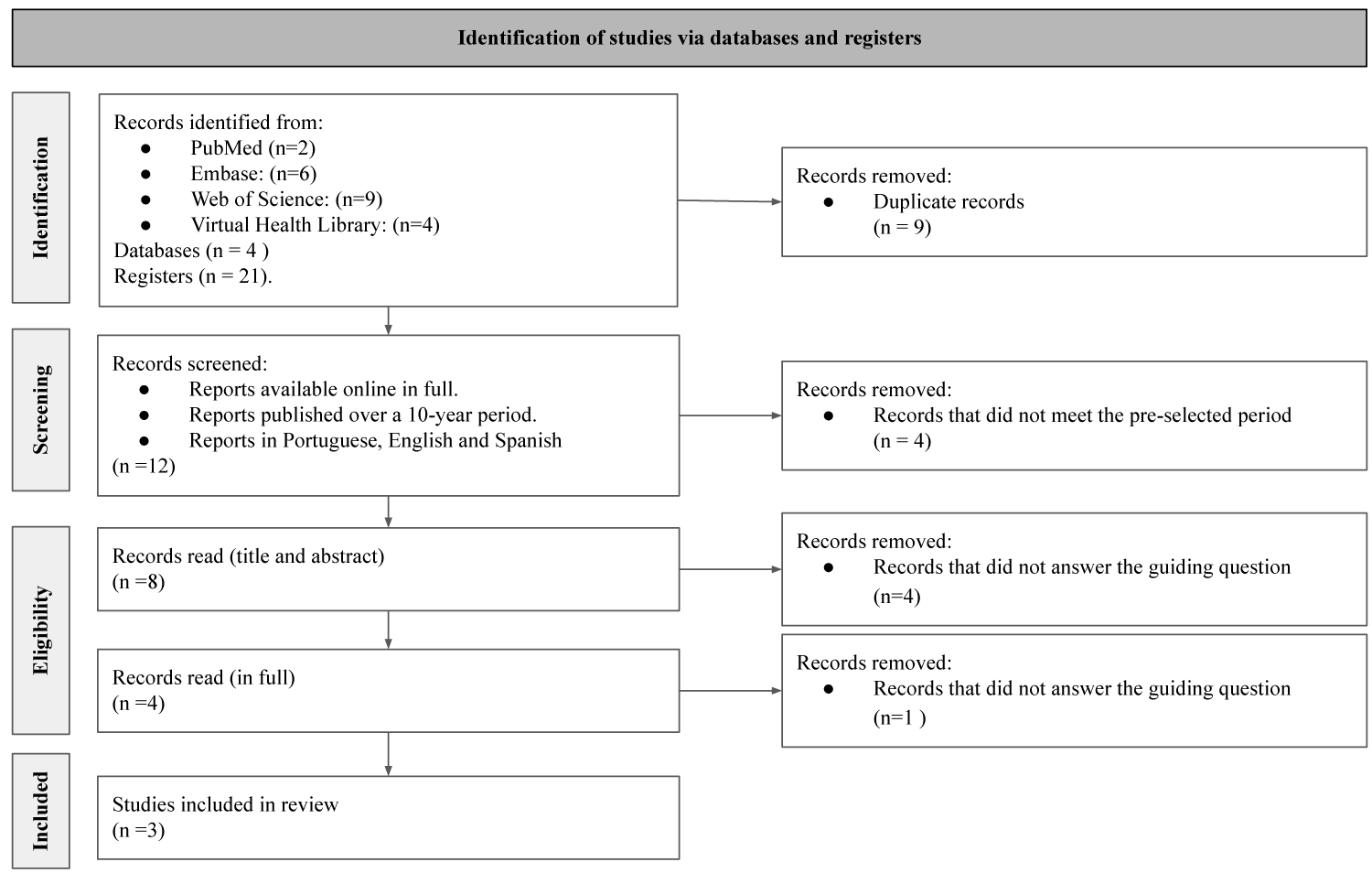
After crossing the descriptors in the chosen databases, and applying the selection and eligibility criteria using the flowchart recommended by the PRISMA methodology (Figure 1), the remaining papers were read in full. In this last step, it was even possible to exclude a study in which aminonitriles were only used as intermediates in the reactions, therefore, they were not the molecules on which the cytotoxicity tests were carried out [11]. Finally, the three works that make up the sample of this review work were submitted to the final process of data collection and analysis.
Figure 1: Study selection flowchart. Source: Own authorship, 2022.
After all the steps for the identification of studies, only three were added, which demonstrates a low amount of scientific investigations that address the cytotoxic effects of aminonitriles. Furthermore, it was also noticed that most of the removed articles were about new methods of obtaining these compounds, in which cytotoxicity assays were not reported.
Table 1 presents the synthesis of the data extraction strategy of the articles included in the review, describing the objectives of the studies and the main results from the application of the methodologies and cells selected for the evaluation of cytotoxicity of the selected aminonitriles.
| Table 1: Papers selected for analysis. | |||
| ID | 1 | 2 | 3 |
| Title | Synthesis and biological evaluation of 4,5,6,7tetrahydrothieno[2,3-c]pyridine–based β-aminonitriles and their derivatives: β-amino carboxamides, (thio)ureas, and tetracycles | Design, synthesis, and evaluation of new α-aminonitrile-based benzimidazole biomolecules as potent antimicrobial and antitubercular agents | Preparation of renieramycin left-half model compounds |
| Authors | Madácsi, et al. 2019 | Shaikh, et al. 2017 | Nakai, et al. 2014 |
| Aminonitriles | 9 different aminonitriles (β-aminonitriles based on 4,5,6,7-tetrahydrothieno[2,3-c]pyridine) | 12 aminonitrilas distintas (sintetizadas a partir da condensação de três componentes de 4-[(1Hbenzimidazol-2-il)metoxi]benzaldeído, anilinas substituídas e cianeto de sódio em acetonitrila) | 12 different aminonitriles left half model of renieramycins and their diastereoisomers, synthesized from phenylalanine derivatives |
| Type of cells on which compounds were tested | Human lung adenocarcinoma (lineage A549), human leukemia (lineage K562) and primary human fibroblasts | Vero cells | Human colon carcinoma (HCT116 lineage), human lung carcinoma (QG56 lineage), and hormone-dependent prostate adenocarcinoma (LNCaP) (DU145 lineage) |
| Method | Fluorimetric assays by reduction of resazurin | Colorimetric test by MTT reduction | Colorimetric test by MTT reduction |
| Result | Of the 9 Gewald aminonitriles tested, only two (16 and 17) exerted moderate cytotoxic activity against cancer cells. | All compounds showed a low level of cytotoxicity against Vero cells. | Of the 12 aminonitriles tested, all showed a cytotoxic effect against the cancer cell lines tested. |
| Level of evidence | 2 | 2 | 2 |
| Source: Own authorship, 2022. | |||
All studies included in this review were published in English and scientific journals dealing with pharmacology or chemistry. Furthermore, the central scope of the works was to obtain a new protocol for the synthesis of aminonitriles, while cytotoxicity tests were performed as secondary objectives. In addition, there was a preference for selecting several molecules other than heterocyclics, but with similar origins and production processes, originating from the same precursor compound, to which radicals and different constituents were added to obtain different molecules.
In the work developed by Madácsi, et al. a fluorimetric resazurin reduction assay was performed as a methodology for evaluating the cytotoxic effects in cancer cell lines and against healthy fibroblastic cells. Furthermore, in the studies by Shaikh, et al. and Nakai, et al. the colorimetric MTT assay was used to estimate the cytotoxic effects on Vero cells and cells of different cancerous lineages [12-14].
Regarding the quantification of the cytotoxic activity of the tested compounds, in the study by Madácsi, et al. cell viability was calculated concerning untreated control cells and blank wells containing medium without cells, measuring the values of half of the maximum inhibitory concentration presented [13]. In this same perspective, in the research by Shaikh, et al. the compound was considered toxic if it caused more than 50% inhibition of normal cells at a concentration 10 times greater than its minimum inhibitory concentration value against the studied microorganisms [14]. Concerning the article published by Nakai, et al. this evaluation was performed from the measurement of the maximum inhibitory concentration of 50% and 80% [12].
In view of the cytotoxic activity of the evaluated molecules, it was observed that in the study developed by Madácsi, et al. of the nine aminonitriles tested, only two (substances 16 and 17), containing methyl grouping in their radicals, showed moderate effects, but compound 16 was more toxic to tumor cells than to fibroblast cells. In the work by Shaikh, et al. all bioactive exhibited low levels of cytotoxicity, with a percentage of Vero cell survival in the range of 17% to 97% [13,14]. In the article published by Nakai, et al. of the 12 aminonitriles evaluated, all were effective against the cancer cell lines tested, with compound 10a achieving a relatively better result than the others concerning human colon carcinoma cell lines (HCT116), as well as Renieramycin M, which showed considerable response to all three of these cancer cell lines [12].
In the evaluations developed by Madácsi, et al., the compounds began to show increasing toxicity when methyl radicals were added to their aromatic ring, two in compound 16 and four in 17 [13]. In studies by Shaikh, et al., the molecules obtained some cytotoxic effect against Vero cells when chlorine atoms were added to the phenyl ring linked to the amine [14]. In the work by Nakai, et al., compounds of the 6,11a-cis configuration model (9a to 12a) were slightly more cytotoxic than the corresponding trans-series [12]. Thus, according to the aforementioned studies, it can be seen that the presence of the methyl group, chlorine atoms, and the trans geometric configuration is encoded as factors that may predispose to cytotoxicity.
In short, of the 33 aminonitriles tested, 21 did not show relevant cytotoxic effects, that is, they have low or even no significant cytotoxic effect, while 12 aminonitriles showed relevant cytotoxicity against cancer cells, especially those presented by the study by Nakai, et al. [12].
Finally, the reliability of the methodologies adopted and the results obtained by the articles selected through AHRQ [9] categorization was scored, in which all articles obtained evidence level 2 because they are experimental studies carried out in vitro.
Research and drug development are based on steps that accumulate knowledge about a particular molecule of interest in the clinical field. Such steps are commonly called pre-clinical and clinical, which seek to demonstrate that the substance of interest is safe, effective, and has the quality to be used as an active ingredient in new drugs [15].
Intending to identify new compounds for use in medicines, recent studies have proposed the use of aminonitriles in addition to their role as intermediates in obtaining substances that are known to be used in the pharmaceutical industry. Such works suggest the use of these substances as pharmacologically active biocompounds given the various reports in the scientific literature regarding the therapeutic potential against various microorganisms, parasites, and even tumor cells [4].
In this sense, the importance of evaluating the safety of these compounds with potential for the development of new therapies becomes evident, since the characterization of in vitro cytotoxicity, a common practice in the biological evaluation of health products, is fundamental for the initial analysis of their biocompatibility [16]. Thus, cytotoxicity tests are based on the exposure of a cell lineage culture to direct or indirect contact with a given substance or target material for analysis, observing the cell changes resulting from the interaction by time and appropriate conditions of exposure with the same [5].
In the works included in this review, tests were used in normal human cells, cancer cells, and also in Vero cells. It is important to note that the screening of candidates for bio actives in vitro allows a faster and more economical evaluation compared to models [17]. However, animal models share greater similarities with the complex living tissues of humans and tests support a greater source of information to measure the safety and efficacy of drugs [18]. Despite this, no articles were found with studies on the cytotoxic effects of aminonitriles, which would be pertinent.
The methods for evaluating the cytotoxic effects used in the analyzed studies were the fluorimetric resazurin reduction assay and the colorimetric tetrazolium assay also called the MTT assay. In this regard, resazurin reduction assays have an advantage over MTT assays, as they are relatively cheaper, use a homogeneous format, and are more sensitive [19]. Furthermore, it is perceived that there is a wide possibility of cytotoxicity tests yet to be explored in the evaluation of aminonitriles, such as those performed in human erythrocytes to verify the hemolytic potential, hemagglutination and the potential for osmotic fragility, which have already been used in biocompatibility studies [20,21].
In the works by Madácsi, et al. and Shaikh, et al., the studied aminonitriles have low or even no significant cytotoxicity, demonstrating the promising potential for the pharmaceutical branch over its use as an antimicrobial drug [13,14]. In the study by Madácsi, et al. those compounds that showed some cellular effect on malignant cell lines were also toxic in healthy fibroblasts, but with lesser effect, demonstrating a certain selectivity [13]. Furthermore, the work by Nakai, et al. achieved great success in their investigation into the possibility of using the selected heterocycles as bioactive in drugs, since the molecules studied had significant antitumor activity [12].
Given the evidence summarized in this integrative literature review, new possibilities were observed in the scientific field for the use of aminonitriles. In general, it was seen that the results of the studies analyzed are quite promising in terms of antibacterial, antifungal, and antitumor activity, but cytotoxic analysis is still scarce. The data indicate the possibility of low toxic cellular capacity or even no significant cytotoxic effect, demonstrating potential in the pharmaceutical field.
Despite the reduced number of studies in the final sample, the level of evidence was considered good, due to the meticulous methodological process applied. However, there is a noticeable need for research that explores cellular effects more specifically, evidencing the mechanism of molecular interactions found in the various areas of pre-clinical investigations, both in vitro and in vivo. In addition, the need to expand cytotoxicity tests is evident, with the use of other protocols and the use of other cell lines, corroborating the construction of more solid information about this class of biomolecules.
Finally, studies mentioning the cytotoxic activities of aminonitriles are scarce. However, those that had this purpose and were presented in this work demonstrated their high structural and chemical versatility, in which the addition of different radicals, such as methyl or chlorine, and stereochemical dispositions appear to influence the cellular response.
Through this review of the current literature, it is expected to contribute to the increase and dissemination of information about this class of molecules, in addition to stimulating new experimental scientific studies, given the need to explore more about their cytotoxic effects.
Financial support
This work was funded by the Infrastructure Program for Young Researchers (First Projects Program - PPP), instituted by Public Notice No. 010/2021 (Term of grant: 3204/2021), by the Research Support Foundation of the State of Paraíba (FAPESQ-PB), in partnership with the National Council for Scientific and Technological Development (CNPq), by the Institutional Program of Scientific Initiation Scholarships (PIBIC) – PIBIC/CNPq 2022/2023 and the Volunteer Institutional Scientific Initiation Program (PIVIC) of the Federal University of Campina Grande (UFCG).
- Kouznetsov VV, Galvis CEP. Strecker reaction and α-amino nitriles: Recent advances in their chemistry, synthesis, and biological properties. Tetrahedron. 2018 Feb 22; 74(8):773–810.
- Grundke C, Vierengel N, Opatz T. α -Aminonitriles: From Sustainable Preparation to Applications in Natural Product Synthesis. Chem Rec. 2020 Sep;20(9):989-1016. doi: 10.1002/tcr.202000066. Epub 2020 Jul 24. PMID: 32706179.
- Shalayel I, Coulibaly S, Ly KD, Milet A, Vallée Y. The Reaction of Aminonitriles with Aminothiols: A Way to Thiol-Containing Peptides and Nitrogen Heterocycles in the Primitive Earth Ocean. Life (Basel). 2018 Oct 19;8(4):47. doi: 10.3390/life8040047. PMID: 30347745; PMCID: PMC6316830.
- Almeida HM, Oliveira I, Ferreira S. Aminonitrile Potential in Terms of Pharmacological and Clinical Applicability. 2021 Feb 21. https://sciforum.net/paper/view/9258
- Masson AO, Nascimento MHM, Lombello CB. Análise comparativa de diferentes métodos de citotoxicidade in vitro. In: XXIV Congresso Brasileiro de Engenharia Biomédica – CBEB. Uberlândia; 2014; 2484-2488.
- Andrade EL, Bento AF, Cavalli J, Oliveira SK, Schwanke RC, Siqueira JM, Freitas CS, Marcon R, Calixto JB. Non-clinical studies in the process of new drug development - Part II: Good laboratory practice, metabolism, pharmacokinetics, safety and dose translation to clinical studies. Braz J Med Biol Res. 2016 Dec 12;49(12):e5646. doi: 10.1590/1414-431X20165646. PMID: 27982281; PMCID: PMC5188860.
- Dhollande S, Taylor A, Meyer S, Scott M. Conducting integrative reviews: a guide for novice nursing researchers. J Res Nurs. 2021 Aug;26(5):427-438. doi: 10.1177/1744987121997907. Epub 2021 Aug 5. PMID: 35251272; PMCID: PMC8894639.
- Stern C, Jordan Z, McArthur A. Developing the review question and inclusion criteria. Am J Nurs. 2014 Apr;114(4):53-6. doi: 10.1097/01.NAJ.0000445689.67800.86. PMID: 24681476.
- Agency for Heathcare Research and Quality. AHRQ Indicators. Agency for Heathcare Research and Quality. [cited 2022 Sep 13]. https://qualityindicators.ahrq.gov/
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. doi: 10.1136/bmj.n71. PMID: 33782057; PMCID: PMC8005924.
- Graphpad Software. Prism. Prism. https://www.graphpad.com/scientific-software/prism/
- Nakai K, Kubo K, Yokoya M, Saito N. Preparation of renieramycin left-half model compounds. Tetrahedron. 2014 Sep 16; 70(37):6529–45.
- Madácsi R, Traj P, Hackler Jr L, Nagy LI, Kari B, Puskás LG, Kanizsai I. Synthesis and biological evaluation of 4,5,6,7-tetrahydrothieno[2,3-c]pyridine–based β-aminonitriles and their derivatives: β-amino carboxamides, (thio)ureas, and tetracycles. Journal of Heterocyclic Chemistry. 2020; 57(2):635–52.
- Shaikh IN, Hosamani KM, Kurjogi MM. Design, synthesis, and evaluation of new α-aminonitrile-based benzimidazole biomolecules as potent antimicrobial and antitubercular agents. Arch Pharm (Weinheim). 2018 Feb;351(2). doi: 10.1002/ardp.201700205. Epub 2018 Jan 22. PMID: 29356105.
- Lupatini E de O, Barreto JOM, Zimmermann IR, Silva EN da. Medicamentos e pesquisa translacional: etapas, atores e políticas de saúde no contexto brasileiro. Saúde debate. 2020 Feb 10;43:181–99.
- Masson AO, Lombello CB. Cytotoxic evaluation methodologies: comparative study according to exposure time. In Foz do Iguaçu; 2016; 1–12. http://slabo.org.br/cont_anais/anais_9_colaob/manuscript/13-032TT.pdf
- Dobrovolskaia MA, McNeil SE. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J Control Release. 2013 Dec 10;172(2):456-66. doi: 10.1016/j.jconrel.2013.05.025. Epub 2013 Jun 3. PMID: 23742883; PMCID: PMC5831149.
- Garattini S, Grignaschi G. Animal testing is still the best way to find new treatments for patients. Eur J Intern Med. 2017 Apr;39:32-35. doi: 10.1016/j.ejim.2016.11.013. Epub 2016 Dec 1. PMID: 27916437.
- Assay Guidance Manual. https://ncats.nih.gov/expertise/preclinical/agm
- de Araújo JSC, de Castilho ARF, Lira AB, Pereira AV, de Azevêdo TKB, de Brito Costa EMM, Pereira MDSV, Pessôa HLF, Pereira JV. Antibacterial activity against cariogenic bacteria and cytotoxic and genotoxic potential of Anacardium occidentale L. and Anadenanthera macrocarpa (Benth.) Brenan extracts. Arch Oral Biol. 2018 Jan;85:113-119. doi: 10.1016/j.archoralbio.2017.10.008. Epub 2017 Oct 14. PMID: 29054025.
- Valáriková J, Čížová A, Račková L, Bystrický S. Anti-staphylococcal activity of quaternized mannan from the yeast Candida albicans. Carbohydr Polym. 2020 Jul 15;240:116288. doi: 10.1016/j.carbpol.2020.116288. Epub 2020 Apr 18. PMID: 32475569.