More Information
Submitted: June 24, 2023 | Approved: July 05, 2023 | Published: July 06, 2023
How to cite this article: Gomes MF, Araújo GR, Silva ZDS, de Sousa RMF, Dias HJ. Identification and Infrared Spectroscopic Study of Lapachol, β-Lapachone and Hydroxy-hydrolapachol. Arch Pharm Pharma Sci. 2023; 7: 028-035.
DOI: 10.29328/journal.apps.1001041
Copyright License: © 2023 Gomes MF, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and repro-duction in any medium, provided the original work is properly cited.
Keywords: Ipê-roxo; Metabolites; FTIR; Elucidation
Identification and Infrared Spectroscopic Study of Lapachol, β-Lapachone and Hydroxy-hydrolapachol
Miquéias Ferreira Gomes1 , Ginandrya Rodrigues Araújo1
, Ginandrya Rodrigues Araújo1 , Ziom Drak Souza Silva1
, Ziom Drak Souza Silva1 , Raquel Maria Ferreira de Sousa2
, Raquel Maria Ferreira de Sousa2 and Herbert Júnior Dias1*
and Herbert Júnior Dias1*
1Núcleo de Química, Instituto Federal de Educação, Ciência e Tecnologia Goiano Campus Urutaí, Urutaí – GO, Brazil
2Instituto de Química, Universidade Federal de Uberlândia, Uberlândia – MG, Brazil.
*Address for Correspondence: Herbert Júnior Dias, Núcleo de Química, Instituto Federal de Educação, Ciência e Tecnologia Goiano Campus Urutaí, Urutaí – GO, Brazil, Email: [email protected]
Metabolites of Brazilian Cerrado species are considered an immense font of biologically active compounds The diversity of organic compounds generated by the secondary metabolism of various Cerrado plants draws attention especially because many of these compounds have the capacity to be structurally modified and, consequently, produce other very interesting derivatives for pharmacological purposes. Despite this, little is described in the literature about fast, easy, and accessible identification methods for any laboratory, such as infrared spectroscopy. In this sense, this work demonstrates the synthesis and elucidation through spectroscopic techniques of lapachol and its synthetic derivatives. Through quick and simple extractions or reactions, lapachol, β-lapachone, and hydroxy-hydrolapachol were obtained with adequate yields. From this, the main FTIR absorptions of the mentioned naphthoquinones are described, which facilitates the identification of these metabolites with high biological potential. The present work contributes could become a simpler source of data for extraction, synthesis, and spectroscopic characterization by FTIR of the compounds.
Brazilian Cerrado is the second largest Brazilian biome, occupying about 21% of the country’s area in the plateau of the South American continent [1,2]. The biome presents an interesting diversity in both fauna and flora, showing an uncountable number of native species [3]. The biome is widespread in the Brazilian territory, occupying the largest areas in the states Federal District, Goiás, Tocantins, Maranhão, Mato Grosso do Sul and Minas Gerais. However, the prevention of deforestation or even government inactions to decrease the devastation of the biome, mainly in the past four years have been impacting subsequently the preservation of native species [3,4].
Particularly, some species of plants are native to the biome Cerrado, being considered a natural marker of it, such as the presence of Tabebuia heptaphylla (Veloso) Toledo, called popularly in Brazil as “Ipê-roxo”. The specie from the Biognoniaceae family is characterized by a crooked trunk, measuring up to 35 meters tall, with opposite typed leaves usually five-seven leaflets, and tubular showy flowers lilac to pink colored [5]. In addition, some Tabebuia spp. have been described in the literature, differing visually by the color of flowers, such as white (Tabebuia roseoalba), yellow (Tabebuia alba), pink (Tabebuia impetiginosa) and others [5,6]. As commonly observed in Cerrado plants, T. heptaphylla is known for its uses in ethnopharmacology, being considered a “wonder drug” for some natives in Brazil and other South American countries, including Argentina, Paraguay, Bolivia, and Peru for some preparations [7-9]. The presence of special metabolites such as flavonoids, napthoquinones, furanonapthoquinones, quinines, benzoic acids, and others can be pointed out as one of the reasons why the use in traditional medicine is commonly related in native populations on the American continent [10].
Due to the presence of remarkable substances made by secondary metabolism, T. heptaphylla is described in the literature with a great range of biological responses. Some of that biological properties have been described for Tabebuia avellanedae Lorentz ex Grisebach or Tecoma impetiginosa Martius, being both botanical sinonimum of T. heptaphylla [5]. El-Hawary and coworkers reviewed the biological responses as well as the presence of substances catalogued in Tabebuia spp [11]. Some parts of the plant, extracts, oils, resins and other preparations have been used, exhibiting anti-inflammatory [12-14], anti-ulcer [15,16], wound healing activity [17], antinociceptive [18], anti-obesity [19,20], antidepressant [21], antimicrobial [22-24], antileishmanial [25], antiviral [26,27], insecticidal [28], anti-oxidant [29], cytotoxic [30,31] and among others.
In major of Tabebuia spp. we observed the presence of naphthoquinones, special metabolites class of organic compounds that have important antioxidant and electrolytic effects, especially by the electrophilic, oxidant, and acid-base properties of a conjugated benzoquinolinic moiety present in them [32,33]. The main naphthoquinone considered a chemical marker of Tabebuia spp. is Lapachol (Scheme 1, (1)), a 1,4 naphthoquinone, which has been described in the literature in at least fifteen species [11]. Lapachol is commonly obtained by extraction from parts of the wood or bark [34,35], and gained prominence after several studies of the substance and the semi-synthetic derivatives obtained over the years against tumor and cancer cell lines in both in vitro and in vivo investigations [36]. In a recent study conducted by Oliveira and coworkers [37], the authors prepared Lapachol-Ru(II)/diphosphine complexes and tested with success the substances against breast cancer, becoming Lapachol’s complex an interesting drug candidate for cancer treatments [37]. Similar results had described for the main derivative, β-Lapachone (Scheme 1, (2)), which displays similar or better actions against cancer cell lines, being used in anticancer agents [38,39].
Scheme 1: Synthesis of β-lapachone (2) and hydroxy-hydrolapachol (3) from lapachol (1).
Considering the remarkable importance of Lapachol and its metabolites, a low-cost, fast, and robust analytical method is necessary to identify the substances. In this sense, Fourier transformed infrared (FTIR) technique has been employed in the analysis of natural products, pharmaceutical substances, industrials, and research laboratories as protocol, mainly due to the fast simpler analysis, with a small amount of sample, being non-destructive technique [40,41]. In addition, the use of FTIR as a technique to identify functional groups and moieties or bonding groups in natural products is done by quantized energy absorption in natural vibrations for each molecular arrangement, becoming especially Mid-Infrared (MIR) an important tool that can be used as a fingerprint for identification of organic compounds [41,42]. Although MIR data had been used as a tool for Lapachol derivatives identification, is observed a clear difficulty to corroborate the experimental information of the structures with data in the literature, becoming the simple analysis by FTIR of its derivatives a tortuous and difficult mission. In this sense, we proposed here a standardization and study of Lapachol and derivatives by MIR as a way to help the rapid identification of these molecules.
Extraction and synthesis of lapachol derivatives
The tree heartwood sawdust of T. heptaphylla (Veloso) Toledo was collected in the city of Ipameri, state of Goiás, Brazil (48° 9’ 36.0’’S 17°44’20.7’’ W), identified and deposited in the Herbarium of Núcleo de Biologia, at Instituto Federal de Educação, Ciência e Tecnologia Goiano Campus Urutaí, Urutaí, Goiás, Brazil. The extraction of lapachol (1) (Scheme 1) occurred as a modified methodology proposed in previous studies of the literature [43]. The bark collected was dried in the stove, and 456 grams of the powder was suspended in a 2 L beaker flask with 1 L of sodium carbonate solution (1% m/v, Sigma-Aldrich, St. Louis, MO, USA). The mixture was stirred for 45 minutes, obtaining after filtration a red-colored solution. To the crude mixture was added HCl 6.0 mol L-1 (Neon, Suzano, SP, Brazil) up to the solution became yellow-colored and started the precipitation process (pH 2.0).
The precipitate was filtered off under vacuum filtration and recrystallized in dichloromethane P.A. (Dinâmica Química, São Paulo, SP, Brazil) and dry under the stove (80 °C, 1 h), obtaining 33.5 g of yellow crystals (7.3 % yield). The structure (1) was confirmed by NMR analysis.
Compound (2) (Scheme 1) was synthesized as previously described by Alves and coworkers [44], where β-lapachone was obtained by reacting 484 mg of (1) with 5.0 mL of sulphuric acid P.A. (Sigma-Aldrich, St. Louis, MO, USA) in a roam-bottomed flask under stirring for 15 minutes. The mixture was vested in 100 mL of cold distilled water (4 °C), obtained by vacuum filtration, and dried in a stove (60 °C) with 376 mg of an orange solid (77.7% yield). Synthetical obtainment of hydroxy-hydrolapachol (3) was performed as described by Petit & Houghton, [45] by a base-catalyzed Michael-type reaction where 250 mg of (2) reacts with 18.0 mL of sodium hydroxide solution 5% (m/V) (Sigma-Aldrich, St. Louis, MO, USA) in a roam-bottomed flask under heating (85 °C to 120 °C) and stirring for 10 minutes. The mixture was cooled and neutralized with HCl 6 mol L-1 up to color change from red-brown to a yellow-colored mixture. The interest compound was obtained by a partition with ethyl acetate P.A. (3 x 15 mL) (Dinâmica Química, São Paulo, SP, Brazil), the organic layer was dried with magnesium sulfate, rotavap under reduced pressure, giving 174 mg of a yellow solid hydroxy-hydrolapachol (64.7% yield). All structures (2) and (3) were confirmed by NMR analysis.
NMR and infrared analysis
All 1H and 13C NMR analyses were performed on a Bruker DRX400 spectrometer (Karlsruhe, Germany, 400 MHz for 1H and 100 MHz for 13C). The 1H and 13C chemical shifts (δ) were described in terms of parts per million (ppm), using tetramethylsilane (TMS, 0.03% v/v) as an internal reference. The experiments were performed at 300 K, in sample concentrations of 10 mL mL-1, in CDCl3 (99.8 atom % D, Sigma-Aldrich, St. Louis, MO, USA). FT-IR analysis was performed in the mid-infrared region in solid state KBr (Shimadzu Scientific, spectroscopic grade, Kioto, Japan) pellet using an infrared spectrometer Shimadzu Affinity-1 model (Shimadzu Corporation, Kioto, Japan). We achieved the region of 4000 to 400 cm-1 with 16 scans per experiment, as previous methodologies reported in the literature [46,47]. All FTIR data were analyzed on Origin program version 6.1 (OriginLab Corporation, Northampton, MA, USA) [48].
NMR analysis
The characterization of the synthesized compounds was achieved by 1H and 13C NMR experiments, described above, and the spectra of (1-3) are in agreement with the chemical shifts as previously published in the literature [49,50].
2-hydroxy-3-(3-methylbut-2-en-1-yl) naphthalene-1,4-dione (lapachol, (1)), yellow powder, mp 137-139 °C, 1H NMR (400 MHz, CDCl3) δ 1.61 (s, 3H), 1.71 (s, 3H), 3.23 (d, 2H J = 7.4 Hz), 5.13 (tt, 1H J = 7.4, J = 1.4 Hz), 7.62 (m, 2H), 7.62 (2H, m); 13C NMR (100 MHz, CDCl3) δ 17.9, 22.6, 25.7, 119.7, 123.5, 126.0, 126.7, 129.4, 132.8, 132.9, 133.8, 134.8, 152.7, 181.7, 184.5.
2,2-dimethyl-3,4-dihydro-2H-benzo[H]chromene-5,6-dione (β-lapachone, (2)), orange solid, mp 153-155 °C, 1H NMR (400 MHz, CDCl3) δ 1.42 (s, 6H),1.84 (t, J = 6.7 Hz, 2H), 2.59 (t, J = 6.7 Hz, 2H), 7.50 (dt, J = 1.1, 7.4 Hz, 1H), 7.66 (dt, J = 1.3, 7.4 Hz, 1H), 7.81 (dd, J = 1.1, 7.7 Hz, 1H), 8.00 (dd, J = 1.4, 6.9 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 17.8, 22.6, 25.7, 119.6, 123.5, 126.0, 126.8, 129.4, 132.8, 132.9, 133.8, 134.8, 154.7, 179.7, 184.5.
4-hydroxy-3-(3-hydroxy-3-methylbutyl) naphthalene-1,2-dione (hydroxy-hydrolapachol, (3)), yellow solid, mp 126-128 °C, 1H: δ 1.41 (s, 6H), 1.85 (t, J = 6.7 Hz, 2H), 2.58 (t, J = 6.7 Hz, 2H), 7.53 (dt, J = 1.1, 7.4 Hz, 1H), 7.61 (dt, J = 1.3, 7.4 Hz, 1H), 7.82 (dd, J = 1.1, 7.7 Hz, 1H), 8.01 (dd, J = 1.4, 6.9 Hz, 1H); 13C: δ 16.8, 26.8, 31.7, 79.4, 112.9, 124.1, 128.7, 130.3, 130.7, 132.8, 134.8, 154.7, 162.1, 178.7, 180.1, 184.5.
Mid-infrared study of lapachol, β-lapachone and hydroxy-hydrolapachol
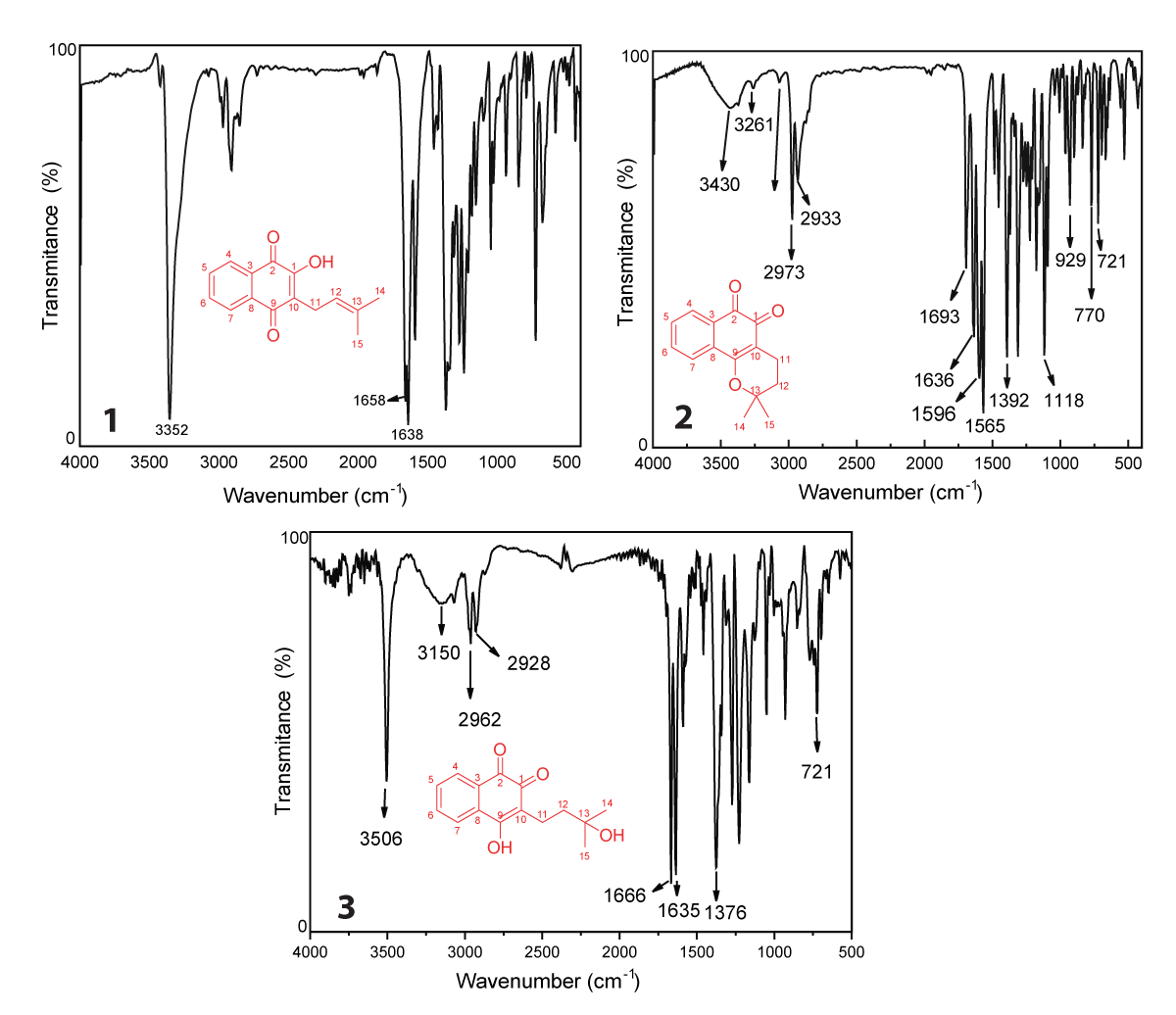
FTIR analysis using specific frequencies on the infrared region to promote absorptions in quantized energy content, promoting stretching and bending of the bonds in befitting natural vibrations, bond types, functional groups, and special molecular arrangements, promoting proven structural elucidation by spectra analysis [51]. Lapachol is characterized by an intense and broadband in 3352 cm-1, common in O-H bonds by symmetrical stretching. Hydrogen-oxygen bonds are observed particularly in regions between 3500 cm-1 and 3100 cm-1, present in some natural products which exhibit an α,β-unsaturated O-H bond, such as flavonoids and naphthoquinones [52,53]. In addition, in a similar region, the symmetrical and asymmetrical stretching in the region between 3100 - 2700 cm-1 are diagnostic of the C–H bond in sp2 and sp3 carbon atoms. In lapachol, we observed depicted in Figure 1.1 the bands referred to C-H bonds in an aliphatic moiety and in the benzenic structure of naphthoquinone, like other natural products which exhibit C-H bonds in structure [54].
We still emphasize the presence of two characteristic bands in 1658 and 1638 cm-1, which distinguishes the presence of double carbonyl groups in the C2 and C9 positions (Figure 1.1). The presence of each band is in consonance with the C = O bond between 1655 - 1635 cm-1, which exhibits an α,β-unsaturated ketones of quinolinic moiety [55]. The extended conjugation increases the effects by a keto-enolic effect with a hydroxyl group of enolic moiety in C1 [55,56]. The harmonic bands in the region between 2000 and 1667 cm-1, deserve to be highlighted due to the presence of a double substitution at the aromatic ring (C3 and C8), which corroborates with the ortho-evidenced band of angular stretching observed in 720 cm-1 [55,57]. The aliphatic moiety was still evidenced by the axial stretching in 1589 cm-1, which characters an is methyl propene ramification, in consonance with the literature [51]. In addition, the methyl group can be confirmed by a double band in 1369 and 1350 cm-1 (Figure 1.1), common in geminal moieties as observed in the lapachol structure [55,58]. The presence of geminal groups is common in some isomethylated compounds, such as sesquiterpene hydrocarbons and other natural products, as reported in the literature [58]. In addition, the additional bands as well the characteristic bands discussed above of (1) are in accordance with computational proposes in the literature [59], and are listed in Table 1.
Figure 1: FTIR spectra of lapachol (1), β-lapachone (2) and hydroxy-hydrolapachol (3) in KBr pellet.
| Table 1: Experimental wavenumber (cm-1) data of lapachol (1), β-lapachone (2) and hydroxy-hydrolapachol (3) (KBr pellet). | |||||
| Lapachol | β-lapachone | Hydroxy-hydrolapachol | |||
| ν (cm-1) | Assignment | ν (cm-1) | Assignment | ν (cm-1) | Assignment |
| 3352 | νOH | 3430 | νC=O | 3506 | νC=O |
| 2000-1667 | νC=CArom | 3261 | νC=O | 3150 | νC=O |
| 1658 | νC=O | 2973 | νC-C | 2962 | νC-C |
| 1638 | νC=O | 2933 | νC-C | 2928 | νC-C |
| 1589 | νC=Ctrisubt | 1689 | νC=O | 1666 | νC=O |
| 1369 | νC-CH3 gem | 1636 | νC=O | 1635 | νC=O |
| 1350 | νC-CH3 gem | 1596 | νC=C | 1376 | ωC-H |
| 720 | ΩArom | 1565 | νC=C | 721 | ωArom |
| 1392 | ice-O-C | ||||
| 1118 | νC-O-C | ||||
| 929 770 721 |
ωArom ωArom ωArom |
||||
| Note: V = Stretching, ω = Wagging, Arom = Aromatic ring, Gen = Geminal | |||||
The FTIR of β-lapachone (2), depicted in Figure 1.2 presents a band between 3430 and 3261 cm-1, which proceeds by enolization of carbonyl groups and was fully cited in the literature in both synthetic and isolated molecules [60-62]. The signals at 2933 cm-1 and 2973 cm-1 are characteristic of the stretching of the C sp2–H bond of the naphthoquinone aromatic ring [63]. Aromatic ring resonance confirmation bands in the characteristic ranges similar to lapachol (1), which show signals in the range of 2000 to 1667 cm-1 confirming substitutions in ortho and para positions of the aromatic ring [59]. The bands at 1596 and 1565 cm-1 are characteristic of C = C aromatic bond stretching, common in some classes of natural products, such as flavonoids [52]. In addition, the region between 1400 – 1000 cm-1 is characteristic of asymmetric and symmetric axial stretching of ether, so bands in 1392 and 1118 cm-1 (Figure 1.2) are C-O-C stretchings [64]. The preferred region of IR spectra also shows signals for simple alkanes to be in this same range, according to the literature [59,65]. Table 1 summarizes the bands observed in the spectrum of β–lapachone observed in Figure 1.2.
The FTIR spectrum of hydroxy-hydrolapachol (Figure 1.3) shows an intense band at 3506 cm-1 characteristic of the O–H bond free of intermolecular interaction from the hydrogen in the aliphatic chain of the molecule, followed by a broad band in low intensity at 3150 cm-1 which could be assigned to O–H bond with intermolecular hydrogen bond interaction [66]. In the range between 2962 – 2968 cm-1, we observed stretch bands of Csp2–H and C sp3–H, as proposed for (1), as well as a C–H folding absorptions at 1376 cm-1 corresponding to the methyl group (CH3) of the hydroxy-hydrolapachol [49,51], as detailed described on Table 1.
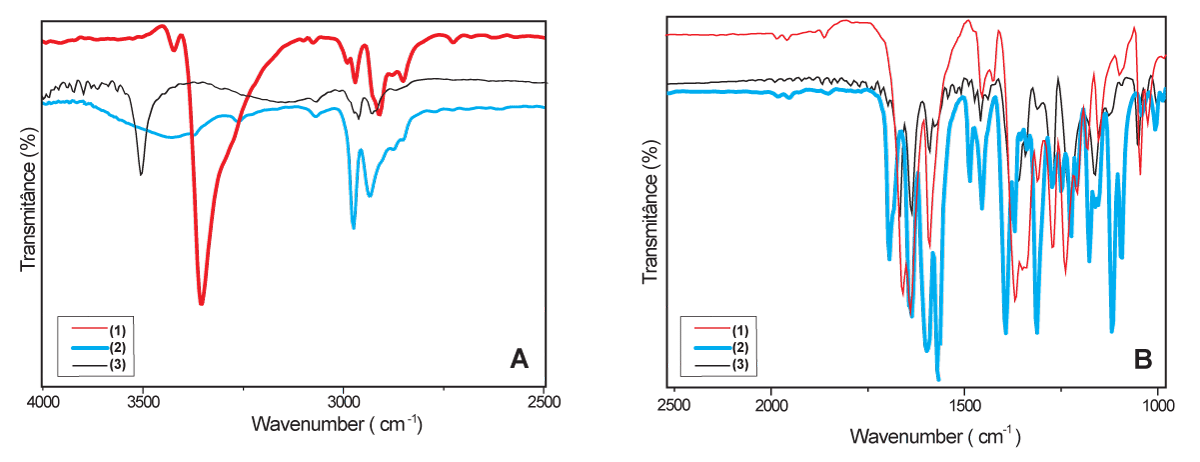
The similarity of hydroxy-hydrolapachol (3) and lapachol (1) undoubtedly makes difficult the distinction between those structures on FTIR spectra, however, some important and interesting details can be used to distinguish the main characteristics of that spectra, as depicted in Figure 2 which shows a comparison between compounds (1 - 3). The confirmation bands of free O–H are present in both, but the hydroxy-hydrolapachol spectra show an O–H intermolecular interaction band between 3500 and 3150 cm-1, which are characteristics of the aliphatic hydroxyl in the structure, as shown on Figure 2A. β-lapachone (2) presents a confirmation band of O–H intermolecular interaction, that can also be influenced by the C = O group band at 3150 cm-1. Another remarkable characteristic that can be highlighted is the less defined bands for Csp3–H by cyclized carbons of the aliphatic radical compared to naphthoquinone compounds (1) and (3) (Figure 2A).
Figure 2: Comparison of FT-IR spectra ranging between 4000 and 2500 cm-1 (A) and ranging between 2500 and 1000 cm-1 (B), of lapachol (1), β-lapachone (2), and hydroxy-hydrolapachol (3) (KBr pellet).
In the region between 2500 to 1000 cm-1, the bands observed for the spectra of (1) and (3) between 2000 and 1666 cm-1 showed similar harmonic signals, characteristic of aromatics substituents, observed in Figure 2B. Considering the substitutions in the aromatic ring being in the same positions for (1) and (3), compound (2) differs in harmonic signal due to the heterocyclic structure linked to the quinone ring. In the regions where carbonyl groups are firmed, the literature predicts higher values for ketones and lower values for others, proving that the energy for this vibration occurs higher for C = O bonds than for C-O-C [55]. Therefore, this is a fundamental point of differentiation between the ether signal in the region of 1390-1100 cm-1 in β-lapachone ((2)), in relation to the ketone vibrations present in the compounds (1) and (3) (Figure 2B). Due to the higher number of signals that occur in the same range, the present work sought to focus on those the main distinction of the compounds, because the structures studied present structural similarities resulting in similar signals with subtle displacements, making this region difficult to analyze.
The extraction of lapachol from sawdust of the ipê tree heartwood was carried out an in according to the literature. From the extracted lapachol, β-lapachone, and hydroxy-hydrolapachol were synthesized with good yield. The main characteristics of the FTIR and 1H and 13C NMR spectra of these compounds were presented and discussed. Lapachol, β-lapachone, and hydroxy-hydrolapachol are substances of great pharmacological potential, and this maintains the growing interest in research and new applications. Ipê-roxo metabolites, as well as several metabolites from plants from the Brazilian Cerrado, have attracted attention due to the wide range of applicability regarding their pharmacological potential. FTIR technique, which is easy, cheap, and fast in its acquisition, does not degrade the sample and allows, through this study, an unequivocal identification of the characteristic bands of the compounds listed above. Thus, this work contributes by being a source of literature that brings together the extraction, synthesis, and spectroscopic characterization of these compounds.
The authors would like to thank Pró-Reitoria de Pesquisa, Pós-Graduação e Inovação – PROPPI-IFGoiano (grant number 23216.000929.2022-22) for the financial support.
ZDSS and GRA worked in obtaining the compounds, in the synthesis of derivatives, and in the analysis of FTIR data. RMFS acted in obtaining the NMR spectra. MFG acted in the conception, writing, and discussion of results arising from this work. HJD worked on the conception, discussion of results, writing of the manuscript, revision, and approval of the final version of the manuscript. All authors agree with the submission of the manuscript and declare that they have not submitted it to another journal during the review process.
- Borlaug NE. Feeding a world of 10 billion people: The miracle ahead. Biotechnology & Biotechnological Equipment. 1997; 11(3–4):3–13. https://doi.org/10.1080/13102818.1997.10818934
- Klink CA, Machado RB. Conservation of the Brazilian Cerrado. Conservation Biology. 2005; 19(3):707–713. https://doi.org/10.1111/j.1523-1739.2005.00702.x
- Ribeiro Neto JA, Pimenta Tarôco BR, Batista Dos Santos H, Thomé RG, Wolfram E, Maciel de A Ribeiro RI. Using the plants of Brazilian Cerrado for wound healing: From traditional use to scientific approach. J Ethnopharmacol. 2020 Oct 5; 260:112547. doi: 10.1016/j.jep.2020.112547. Epub 2020 Jan 7. PMID: 31917276.
- Brasil. Deforestation in the Cerrado in 2018. http://combateaodesmatamento.mma.gov.br/images/Doc_ComissaoExecutiva/Balanco-PPCDAm-e-PPCerrado_2018.pdf
- Carvalho PER. Brazilian Tree Species (1st ed.). Embrapa Technological Information. 2003; 1.
- Grose SO, Olmstead RG. Taxonomic revisions in the polyphyletic genus Tabebuia s. I. (Bignoniaceae). Systematic Botany. 2007; 32(3):660–670. https://doi.org/10.1600/036364407782250652
- Duke JA. Handbook of Medicinal Herbs (2nd ed.). CRC Press. 2002.
- Gómez Castellanos JR, Prieto JM, Heinrich M. Red Lapacho (Tabebuia impetiginosa)-a global ethnopharmacological commodity? J Ethnopharmacol. 2009 Jan 12;121(1):1-13. doi: 10.1016/j.jep.2008.10.004. Epub 2008 Nov 1. PMID: 18992801.
- Sacau EP, Estévez-Braun A, Ravelo AG, Ferro EA, Tokuda H, Mukainaka T, Nishino H. Inhibitory effects of lapachol derivatives on epstein-barr virus activation. Bioorg Med Chem. 2003 Feb 20;11(4):483-8. doi: 10.1016/s0968-0896(02)00542-4. PMID: 12538012.
- Barbosa-Filho JM, Lima CSA, Amorim ELC, Sena KXF, Almeida JRG, da-Cunha EVL, Silva MS, Agra M de F, Braz-Filho R. Botanical study, phytochemistry and antimicrobial activity of Tabebuia aurea. Phyton (Buenos Aires). 2004; 73: 221–228.
- El-Hawary SS, Taher MA, Amin E, Fekry AbouZid S, Mohammed R. Genus Tabebuia: A comprehensive review journey from past achievements to future perspectives. Arabian Journal of Chemistry. 2021; 14(4):103046. https://doi.org/10.1016/j.arabjc.2021.103046
- Lee MH, Choi HM, Hahm DH, Her E, Yang HI, Yoo MC, Kim KS. Analgesic and anti-inflammatory effects in animal models of an ethanolic extract of Taheebo, the inner bark of Tabebuia avellanedae. Mol Med Rep. 2012 Oct;6(4):791-6. doi: 10.3892/mmr.2012.989. Epub 2012 Jul 17. PMID: 22825254.
- Suo M, Isao H, Kato H, Takano F, Ohta T. Anti-inflammatory constituents from Tabebuia avellanedae. Fitoterapia. 2012 Dec;83(8):1484-8. doi: 10.1016/j.fitote.2012.08.014. Epub 2012 Aug 29. PMID: 22955001.
- Zhang L, Hasegawa I, Ohta T. Iridoid Esters from Tabebuia avellanedae and Their In Vitro Anti-inflammatory Activities. Planta Med. 2017 Jan;83(1-02):164-171. doi: 10.1055/s-0042-110322. Epub 2016 Jun 28. PMID: 27352388.
- Pereira IT, Burci LM, da Silva LM, Baggio CH, Heller M, Micke GA, Pizzolatti MG, Marques MC, Werner MF. Antiulcer effect of bark extract of Tabebuia avellanedae: activation of cell proliferation in gastric mucosa during the healing process. Phytother Res. 2013 Jul;27(7):1067-73. doi: 10.1002/ptr.4835. Epub 2012 Sep 12. PMID: 22969019.
- Twardowschy A, Freitas CS, Baggio CH, Mayer B, dos Santos AC, Pizzolatti MG, Zacarias AA, dos Santos EP, Otuki MF, Marques MC. Antiulcerogenic activity of bark extract of Tabebuia avellanedae, Lorentz ex Griseb. J Ethnopharmacol. 2008 Aug 13;118(3):455-9. doi: 10.1016/j.jep.2008.05.013. Epub 2008 May 18. PMID: 18579323.
- Coelho JM, Antoniolli AB, Nunes e Silva D, Carvalho TM, Pontes ER, Odashiro AN. O efeito da sulfadiazina de prata, extrato de ipê-roxo e extrato de barbatimão na cicatrização de feridas cutâneas em ratos [Effects of silver sulfadiazine, ipê roxo (tabebuia avellanedae) extract and barbatimão (stryphnodendron adstringens) extract on cutaneous wound healing in rats]. Rev Col Bras Cir. 2010 Feb;37(1):45-51. Portuguese. doi: 10.1590/s0100-69912010000100010. PMID: 20414576.
- de Miranda FG, Vilar JC, Alves IA, Cavalcanti SC, Antoniolli AR. Antinociceptive and antiedematogenic properties and acute toxicity of Tabebuia avellanedae Lor. ex Griseb. inner bark aqueous extract. BMC Pharmacol. 2001;1:6. doi: 10.1186/1471-2210-1-6. Epub 2001 Sep 13. PMID: 11574048; PMCID: PMC56902.
- Choi WH, Ahn J, Jung CH, Jang YJ, Ha TY. β-Lapachone Prevents Diet-Induced Obesity by Increasing Energy Expenditure and Stimulating the Browning of White Adipose Tissue via Downregulation of miR-382 Expression. Diabetes. 2016 Sep;65(9):2490-501. doi: 10.2337/db15-1423. Epub 2016 May 31. PMID: 27246910.
- Iwamoto K, Fukuda Y, Tokikura C, Noda M, Yamamoto A, Yamamoto M, Yamashita M, Zaima N, Iida A, Moriyama T. The anti-obesity effect of Taheebo (Tabebuia avellanedae Lorentz ex Griseb) extract in ovariectomized mice and the identification of a potential anti-obesity compound. Biochem Biophys Res Commun. 2016 Sep 23;478(3):1136-40. doi: 10.1016/j.bbrc.2016.08.081. Epub 2016 Aug 15. PMID: 27539320.
- Freitas AE, Machado DG, Budni J, Neis VB, Balen GO, Lopes MW, de Souza LF, Veronezi PO, Heller M, Micke GA, Pizzolatti MG, Dafre AL, Leal RB, Rodrigues AL. Antidepressant-like action of the bark ethanolic extract from Tabebuia avellanedae in the olfactory bulbectomized mice. J Ethnopharmacol. 2013 Feb 13;145(3):737-45. doi: 10.1016/j.jep.2012.11.040. Epub 2012 Dec 10. PMID: 23237932.
- Cwikla C, Schmidt K, Matthias A, Bone KM, Lehmann R, Tiralongo E. Investigations into the antibacterial activities of phytotherapeutics against Helicobacter pylori and Campylobacter jejuni. Phytother Res. 2010 May;24(5):649-56. doi: 10.1002/ptr.2933. PMID: 19653313.
- Fernandez A, Cock IE. Tabebuia impetiginosa(Mart. Ex DC. Mattos) bark extracts inhibit the growth gastrointestinal bacterial pathogens and potentiate the activity of some conventional antibiotics. Pharmacognosy Communications. 2020; 10(2):75–82. https://doi.org/10.5530/pc.2020.2.15
- Mehmood Y, Ashraf MI, Rana S, Riaz H, Raza SA, Khan Z. mohy-ud-din. Anti-pseudomonas aeruginosa drug; to evaluate bactericidal activity of Tabebuia Impetiginosa against pseudomonas aeruginosa and its synergistic effect with common antipseudomonas aeruginosa drug prof. The Professional Medical Journal. 2018; 25(10):1574–1580. https://doi.org/10.29309/TPMJ/18.4509
- Ali A, Kiderlen A, Kolodziej H. Lapachol and isomeric 5- and 8-hydroxy-2-(1′-hydroxyethyl) naphtho[2,3-b] furan-4,9-diones are effective antileishmanial constituents of Tabebuia avellanedae. Planta Medica. 2010; 76(12). https://doi.org/10.1055/s-0030-1264769
- Brandão GC, Kroon EG, dos Santos JR, Stehmann JR, Lombardi JA, Braga de Oliveira A. Antiviral activity of Bignoniaceae species occurring in the State of Minas Gerais (Brazil): part 1. Lett Appl Microbiol. 2010 Oct;51(4):469-76. doi: 10.1111/j.1472-765X.2010.02924.x. PMID: 20840554.
- Brandão GC, Kroon EG, Santos JR. dos Stehmann JR, Lombardi JA, Oliveira AB de. Antiviral activities of plants occurring in the state of Minas Gerais, Brazil: Part 2. Screening Bignoniaceae species. Revista Brasileira de Farmacognosia. 2010; 20(5):742–750. https://doi.org/10.1590/S0102-695X2010005000035
- Borges JCM, Haddi K, Oliveira EE, Andrade SB, Nascimento VL, Melo ST, Didonet J, Carvalho JCT, Cangussu AS, Soares IM, Ascencio SD, Raposo NRB, Aguiar RWS. Mosquiticidal and repellent potential of formulations containing wood residue extracts of a Neotropical plant, Tabebuia heptaphylla. Industrial Crops and Products. 2019; 129:424–433. https://doi.org/10.1016/j.indcrop.2018.12.022
- Budni P, Petronilho FC, Citadini-Zanette V, Marcondes C, Zoch AN, Reginatto FH, Dal-Pizzol F. Preliminary studies of the antioxidant activity of adult and young leaf extract hydroetanolic of Tabebuia heptaphylla (Vell.) Toledo (ipê-roxo). Latin American Journal of Pharmacy. 2007; 26(3):394–398.
- Telang N, Nair HB, Wong GYC. Growth inhibitory efficacy and anti-aromatase activity of Tabebuia avellanedae in a model for post-menopausal Luminal A breast cancer. Biomed Rep. 2019 Nov;11(5):222-229. doi: 10.3892/br.2019.1244. Epub 2019 Oct 3. PMID: 31632670; PMCID: PMC6792322.
- Zhang L, Tatsuno T, Hasegawa I, Tadano T, Ohta T. Furanonaphthoquinones from Tabebuia avellanedae induce cell cycle arrest and apoptosis in the human non-small cell lung cancer cell line A549. Phytochemistry Letters. 2015; 11: 9–17. https://doi.org/10.1016/j.phytol.2014.09.013
- Hellwig P. Infrared spectroscopic markers of quinones in proteins from the respiratory chain. Biochim Biophys Acta. 2015 Jan;1847(1):126-33. doi: 10.1016/j.bbabio.2014.07.004. Epub 2014 Jul 12. PMID: 25026472.
- Sousa ET, Lopes WA, Andrade JB de. Sources, formation, reactivity and determination of quinones in the atmosphere. Química Nova. 2016; 39(4):486–495. https://doi.org/10.5935/0100-4042.20160034
- Schmeda-Hirschmann G, Papastergiou F. Naphthoquinone derivatives and lignans from the Paraguayan crude drug "tayï pytá" (Tabebuia heptaphylla, Bignoniaceae). Z Naturforsch C J Biosci. 2003 Jul-Aug;58(7-8):495-501. doi: 10.1515/znc-2003-7-809. PMID: 12939034.
- Yamashita M, Kaneko M, Tokuda H, Nishimura K, Kumeda Y, Iida A. Synthesis and evaluation of bioactive naphthoquinones from the Brazilian medicinal plant, Tabebuia avellanedae. Bioorg Med Chem. 2009 Sep 1;17(17):6286-91. doi: 10.1016/j.bmc.2009.07.039. Epub 2009 Jul 23. PMID: 19674905.
- Epifano F, Genovese S, Fiorito S, Mathieu V, Kiss R. Lapachol and its congeners as anticancer agents: a review. Phytochemistry Reviews. 2014; 13(1):37–49. https://doi.org/10.1007/s11101-013-9289-1
- Oliveira KM, Honorato J, Demidoff FC, Schultz MS, Netto CD, Cominetti MR, Correa RS, Batista AA. Lapachol in the Design of a New Ruthenium(II)-Diphosphine Complex as a Promising Anticancer Metallodrug. J Inorg Biochem. 2021 Jan;214:111289. doi: 10.1016/j.jinorgbio.2020.111289. Epub 2020 Oct 23. PMID: 33137682.
- de Andrade JKF, da Silva Góes AJ, Barbosa VX, de Lima Silva MS, Matos Donato MA, Peixoto CA, Militão GCG, da Silva TG. Anticancer activity of β-Lapachone derivatives on human leukemic cell lines. Chem Biol Interact. 2022 Sep 25;365:110057. doi: 10.1016/j.cbi.2022.110057. Epub 2022 Aug 5. PMID: 35934135.
- Nirmala MJ, Samundeeswari A, Sankar PD. Natural plant resources in anti-cancer therapy-A review. Research in Plant Biology. 2011; 1(3):1–14.
- Baulsir CF, Simler RJ. Design and evaluation of IR sensors for pharmaceutical testing. Advanced Drug Delivery Reviews. 1996; 21(3):191–203. https://doi.org/10.1016/S0169-409X(96)00407-3
- Yap KY-L, Chan SY, Lim CS. Infrared-based protocol for the identification and categorization of ginseng and its products. Food Research International. 2007; 40(5):643–652. https://doi.org/10.1016/j.foodres.2006.11.009
- Tu-ya Yang P, Sun S, Zhou Q, Bao X, Noda I. Analysis of fingerprints features of infrared spectra of various processed products of Radix Aconiti kusnezoffii. Journal of Molecular Structure. 2010; 974(1–3):103–107. https://doi.org/10.1016/j.molstruc.2010.01.008
- Barbosa TP, Neto DH. Preparation of lapachol derivatives in acid and base media: proposed experiments for Organic Chemistry laboratory classes. Química Nova. 2013; 36(2):331–334. https://doi.org/10.1590/S0100-40422013000200021
- Alves GMC, Rolim LA, Neto RPJ, Leite ACL, Brondani DJ, Medeiros FPM, de Bieber LW, Mendonça Junior FJB. Purification and characterization of β-lapachone and stability study of the crystals under different storing conditions. Química Nova. 2008; 31(2):413–416. https://doi.org/10.1590/S0100-40422008000200039
- Pettit GR, Houghton LE. Lapachol. Canadian Journal of Chemistry. 1968; 46(14): 2471–2472. https://doi.org/10.1139/v68-404
- Burgula Y, Khali D, Kim S, Krishnan SS, Cousin MA, Gore JP, Reuhs BL, Mauer LJ. Review of mid-infrared fouritransform-infrared spectroscopy applications for bacterial detection. Journal of Rapid Methods and Automation in Microbiology. 2007; 15(2), 146–175. https://doi.org/10.1111/j.1745-4581.2007.00078.x
- Wartewig S, Neubert RH. Pharmaceutical applications of Mid-IR and Raman spectroscopy. Adv Drug Deliv Rev. 2005 Jun 15;57(8):1144-70. doi: 10.1016/j.addr.2005.01.022. PMID: 15885850.
- Origin Lab Corporation (2001) Origin Pro, Version 6.1 (6.1). Origin Lab Corporation.
- Pettit GR, Houghton LE. Synthesis of hydroxyhydrolapachol and lapachol. Journal of the Chemical Society C: Organic. 1971; 1(0): 509–511. https://doi.org/10.1039/j39710000509
- Salas C, Tapia RA, Ciudad K, Armstrong V, Orellana M, Kemmerling U, Ferreira J, Maya JD, Morello A. Trypanosoma cruzi: activities of lapachol and alpha- and beta-lapachone derivatives against epimastigote and trypomastigote forms. Bioorg Med Chem. 2008 Jan 15;16(2):668-74. doi: 10.1016/j.bmc.2007.10.038. Epub 2007 Oct 18. PMID: 18029184.
- Pavia DL, Lampman GM, Kriz GS, Vyvyan JR. Introduction to Spectroscopy (5th ed.). Cengage Learing. 2011.
- Heneczkowski M, Kopacz M, Nowak D, Kuźniar A. Infrared spectrum analysis of some flavonoids. Acta Poloniae Pharmaceutica. 2001; 58(6): 415–420.
- Rostkowska H, Nowak MJ, Lapinski L, Adamowicz L. Molecular structure and infrared spectra of 2-hydroxy-1,4- naphthoquinone; Experimental matrix isolation and theoretical Hartree–Fock and post Hartree–Fock study. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 1998; 54(8): 1091–1103. https://doi.org/10.1016/S1386-1425(98)00032-8
- Costa RA, da Silva JN, Oliveira KMT, Dutra LM, Costa EV. Quantum chemical studies, vibrational analysis, molecular dynamics and docking calculations of some ent-kaurane diterpenes from Annona vepretorum: a theoretical approach to promising anti-tumor molecules. Structural Chemistry. 2020; 31(3): 1223–1243. https://doi.org/10.1007/s11224-020-01491-2
- Fleming I, Williams D. Infrared and Raman Spectra. In Spectroscopic Methods in Organic Chemistry. Springer International Publishing. 2019; 85-121.https://doi.org/10.1007/978-3-030-18252-6_3
- Benassi R, Ferrari E, Lazzari S, Spagnolo F, Saladini M. Theoretical study on Curcumin: A comparison of calculated spectroscopic properties with NMR, UV–vis and IR experimental data. Journal of Molecular Structure. 2008; 892(1–3): 168–176. https://doi.org/10.1016/j.molstruc.2008.05.024
- Koczón P, Lewandowski W, Mazurek AP. Vibrational (FT-IR and FT-Raman) and NMR studies on selected metal (Ca, Mn, Zn) complexes with ortho-, meta-, and para-iodobenzoic acids. Vibrational Spectroscopy. 1999; 20(2): 143–149. https://doi.org/10.1016/S0924-2031(99)00032-6
- Morin P, Pichard H, Pichard H, Caude M, Rosset R. Supercritical fluid chromatography of sesquiterpene hydrocarbons on silica packed columns with on-line Fourier transform infrared detection. Journal of Chromatography A. 1991; 464: 125–137. https://doi.org/10.1016/S0021-9673(00)94229-8
- Delarmelina M, Ferreira GB, Ferreira VF, de M Carneiro JW. Vibrational spectroscopy of lapachol, α- and β-lapachone: Theoretical and experimental elucidation of the Raman and infrared spectra. Vibrational Spectroscopy. 2016; 86: 311–323. https://doi.org/10.1016/j.vibspec.2016.08.009
- Ilčin M, Holá O, Bakajová B, Kučerík J. FT-IR study of gamma-radiation induced degradation of polyvinyl alcohol (PVA) and PVA/humic acids blends. Journal of Radioanalytical and Nuclear Chemistry. 2010; 283(1): 9-13. https://doi.org/10.1007/s10967-009-0321-2
- Karabulut S, Namlı H. An FT-IR and DFT based new approach for the detection of tautomer proportions in solution. Journal of Molecular Structure. 2012; 1024: 151-155. https://doi.org/10.1016/j.molstruc.2012.05.029
- Rao KS, St-Jean F, Kumar A. Quantitation of a ketone enolization and a vinyl sulfonate stereoisomer formation using inline IR spectroscopy and modeling. Organic Process Research & Development. 2019; 23(5), 945–951. https://doi.org/10.1021/acs.oprd.9b00042
- Cahyana AH, Nazhifah N, Liandi AR. One-pot four-component synthesis of phenazine derivative using 2-hydroxy-1,4-naphthoquinone. IOP Conference Series: Materials Science and Engineering. 2020; 902(1): 012020. https://doi.org/10.1088/1757-899X/902/1/012020
- Kordić B, Kovačević M, Sloboda T, Vidović A, Jović B. FT-IR and NIR spectroscopic investigation of hydrogen bonding in indole-ether systems. Journal of Molecular Structure. 2017; 1144: 159–165. https://doi.org/10.1016/j.molstruc.2017.05.035
- Howard RW, Baker JE, Morgan ED. Novel diterpenoids and hydrocarbons in the Dufour gland of the ectoparasitoid Habrobracon hebetor (Say) (Hymenoptera: Braconidae). Arch Insect Biochem Physiol. 2003 Nov;54(3):95-109. doi: 10.1002/arch.10104. Erratum in: Arch Insect Biochem Physiol. 2004 Feb;55(2):102. PMID: 14571504.
- Kovács A, Keresztury G, Izvekov V. Intramolecular hydrogen-bonding in 2-nitroresorcinol. A combined FT-IR, FT-Raman and computational study. Chemical Physics. 2000; 253(2–3): 193-204. https://doi.org/10.1016/S0301-0104(99)00390-0