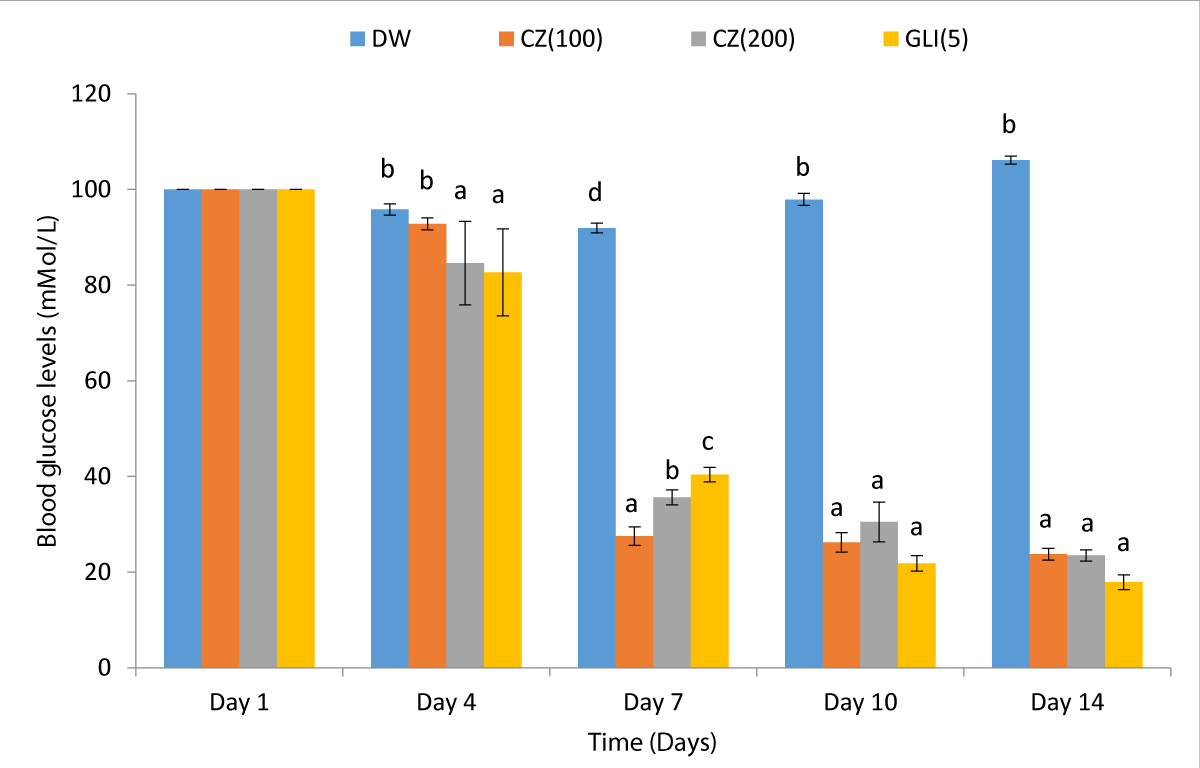More Information
Submitted: June 07, 2023 | Approved: July 12, 2023 | Published: July 14, 2023
How to cite this article: Ayoola MD, Oluwagbemi M, Odediran AS, Oladoja FA, Kasumu OE. Evaluation of the Antihyperglycaemic Activities, Safety and Phytochemical Profile of Celtis zenkeri Engl. Arch Pharm Pharma Sci. 2023; 7: 036-044.
DOI: 10.29328/journal.apps.1001042
Copyright License: © 2023 Ayoola MD, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and repro-duction in any medium, provided the original work is properly cited.
Keywords: Diabetes mellitus; Toxicological studies; Chemical constituents; Celtis zenkeri Engl
Evaluation of the Antihyperglycaemic Activities, Safety and Phytochemical Profile of Celtis zenkeri Engl
Marcus Durojaye Ayoola1*, Akintunde Samuel Odediran1, Samson Oluwaseyi Famuyiwa2, Moyinoluwa Oluwagbemi3, Lateefat Idowu Afolabi3, Farouk Adedeji Oladoja4 and Oluwabusayo Emmanuel Kasumu4
1Department of Pharmacognosy, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria
2Department of Chemistry, Faculty of Science, Obafemi Awolowo University, Nigeria, Ile-Ife, Osun State, Nigeria
3Department of Pharmacognosy, Faculty of Pharmacy, Olabisi Onabanjo University, Sagamu, Ogun State, Nigeria
4Department of Pharmacology and Toxicology, Faculty of Pharmacy, Olabisi Onabanjo University, Sagamu, Ogun State, Nigeria
*Address for Correspondence: Marcus Durojaye Ayoola, Department of Pharmacognosy, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria, Email: [email protected]
Objective: The study evaluated the hyperglycaemia-lowering effects, safety, and phytochemical profile of Celtis zenkeri leaf extract in order to justify its antidiabetic folkloric usage.
Methods: Modified OECD test guidelines were used to assess its acute and sub-acute toxicity while its effect on blood parameters such as blood glucose, and haematological and biochemical levels were evaluated using appropriate assays. Both in vitro and in vivo antihyperglycaemic assays were used for the antidiabetic studies while histology of the pancreas, liver, and kidney of the rats was examined after treatment with the extract at 250, 500, and 1000 mg/kg for 21 days. GC-MS analysis was used to determine the chemical constituents of the extract.
Results: The results obtained showed that the leaf extract of C. zenkeri was not toxic in rats at 5000 mg/kg. It elicited a significant decrease in the blood glucose levels of the animals but did not affect the haematological and biochemical components of normal rats. It significantly inhibited α-amylase and α-glucosidase actions and gave comparable activity to glibenclamide (5 mg/kg) at all time points at 200 and 400 mg/kg. The extract comparably reduced blood glucose levels with glibenclamide at 100 and 200 mg/kg on days 10 and 14 in drug-induced diabetic rats and maintained the histoarchitecture of the liver, kidney, and pancreas at 250 and 500 mg/kg.
Conclusion: The study justified the ethnomedicinal use of C. zenkeri in diabetes management.
Plants have been universally used as therapeutic agents in all the major medical systems, irrespective of the underlying philosophical premise [1] and its use is a long-standing practice, predating written human history [2,3]. Several drugs of importance used in orthodox medicine originated from plants. Some examples include: artemisinin that was isolated from Artemisia annua is effective against a chloroquine-resistant strain of Plasmodium falciparum, digoxin, and digitoxin, with potent cardiotonic activity, were isolated from Digitalis purpurea, tubocurarine, used as a muscle relaxant in surgery (as an adjunct to anaesthesia) and in certain neurological conditions was obtained from curare (Strychnos toxifera), reserpine is used to treat certain cases of mild hypertension and anxiety and was obtained from Rauwolfia vomitoria, galegine from which metformin that is used in type 2 diabetes management was derived was originally isolated from Galega officinale [1,4]. Hence, there is a need for further investigation of medicinal plants for possible newer drugs and/or basis for drug synthesis. Several synthetic drugs that were developed for the treatment of health conditions including diabetes mellitus are very expensive and the associated side effects have limited their affordability, especially in developing countries. This has necessitated the need to consider an alternative method of therapy such as traditional medicine [5].
Celtis zenkeri Engl. (Cannabaceae) commonly known as the West African nettle tree, and ‘Ita-Gidi’ in the Yoruba language, is a deciduous tree that is native to the West African region, including countries such as Ghana, Nigeria, and Cameroon [6]. It is an important plant in traditional medicine having its various parts being used for medicinal purposes. The bark is used to treat fever, malaria, and diarrhea, the leaves are used to treat wounds and skin infections [7] while the fruit is also used to treat constipation [8]. Celtis zenkeri has been found to possess antioxidant [9,10], anti-inflammatory [9], anticancer [11,12], anti-diabetic [13] and antimicrobial [14] activities. The bark contains alkaloids, tannins, and flavonoids, while the leaves contain essential oils, phenolics, and flavonoids [15]. Gas chromatography-mass spectrometry analysis of the essential oils of the stem bark and leaves C. zenkeri identified cycloeicosane, phytol, and α-caryophyllene as the most abundant compounds [10] while zenkeramide was isolated from the stem bark [16]. This study was designed to evaluate the antidiabetic and safety of the methanolic leaf extract of C. zenkeri as well as analyze it for its chemical compounds in order to justify its folkloric use in the management of diabetes mellitus.
Materials and equipment
All the solvents used were of analytical grade. Rotary evaporator (RE301/601/801 model, Yamato Scientific America, Inc., U.S.A), chiller (Churchill, Instrument Co. Ltd, U.K), vacuum pump (MB 338618 model, Edwards High Vacuum Int., England), oven (Hearson & Co. Ltd, London), Mettler electronic weighing balance (AB 54 model, Mettler Toledo, U.S.A), Ultra-violet (UV) lamp (254 and 366 nm) (Grant Instrument, U.K), ACCU-CHEK Glucometer (model GB 11558973, Roche, Germany) with ACCU-CHECK test strips (Roche, Germany), UV spectrophotometer, Dutrao (Model SM 600, Shang Yhai Yong Chuang Medical Instrument Co. Ltd) spetrophotometric microplate reader, Automated haematology analyzer, Centrifuge, Semi-automated biochemistry analyzer. Sodium Citrate, Citric acid, Streptozotocin, Glibenclamide® (Sigma-Aldrich Co. LLC, U.S.A).
Plant materials and extraction
The leaves of Celtis zenkeri were collected at the biological farm of the Obafemi Awolowo University, Ile-Ife, Osun State and authenticated by Mr. Ogunlowo Ifeoluwa at the Faculty of Pharmacy Herbarium with the Herbarium Specimen Number, FPI 2420. The leaves were air-dried, pulverised, and extracted in methanol for 3 days and shaken intermittently. The extract solution was filtered and the marc was re-extracted three times and concentrated in-vacuo to obtain a yield of 14.15% w/w.
Animals
The experiment was conducted on healthy Wistar rats weighing between 120 and 180 g of both sexes that were bred under standard conditions (temp. 27 °C ± 3 °C, relative humidity 65%) at the animal house, Department of Pharmacology, Faculty of Pharmacy, Olabisi Onabanjo University, Sagamu, Nigeria. They were fed on a standard pellet diet (Bendel Feeds, Nigeria) and water was freely given as required.
Acute and subacute toxicity tests
The acute toxicity test was carried out according to the modified OECD Test Guideline 423 [17]. Annex 3 model. Distilled water and single oral administration of 5000 mg/kg extract were administered to two groups of 8 animals each, respectively. They were later observed for signs of gross toxicity, behavioural changes, and mortality, one hour after administration and daily for 14 days [18]. Also, the sub-acute toxicity test was carried out following the modified OECD Test Guideline 407 OECD. [19]. Three graded doses of 250, 500, and 1000 mg/kg of C. zenkeri leaf extract were solubilised in 1% Tween 80 in distilled water and administered to groups of 8 rats daily for 21 days. The blood glucose levels of the rats were monitored on days 1, 7, 14, and 21. The animals were anaesthetized using chloroform and a blood sample (5 mL) was collected by cardiac puncture after the 21st day [19].
Haematological analysis
A blood sample (1.5 mL) was used for the haematological study using the automated haematology analyzer by aspirating about 50 µL of blood into the automated haematology analyzer [20].
Biochemical assays
A blood sample (3.5 mL) was used for biochemical assay using various analyzing kits and a semi-automated biochemical analyser. The serum was analyzed for biochemical markers such as total cholesterol (TC), Alanine transaminase (ALT), Aspartate transaminase (AST), Alkaline phosphatase (ALP), and Creatinine using commercial kits obtained from Randox Laboratories Ltd. (Crumlin, UK) and following the guidelines described by the manufacturer [21].
Histopathological examination
Tissue samples of the pancreas, liver, and kidney were prepared using the method described by Baker and Silverston. The tissue histology slides were viewed under the light microscope at ×400 magnification [22].
Antidiabetic studies
In vitro α-amylase inhibitory activity of the extract: The assay was evaluated using the modified procedure of Bahman, et al. 2008 [23], and Akinwunmi and Ayoola 2018 [24]. A volume of 100 µL of extract or acarbose (positive control) and 100 µL of 0.02 M phosphate buffer (pH 6.9 with 0.006 M sodium chloride) containing α- amylase from Aspergillus oryzae (0.5 mg/mL) were added to each tube and incubated at 25 °C for 10 min. After pre-incubation, 100 µL of 1% starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M sodium chloride) was added to each tube. The reaction was stopped with 200 µL of dinitrosalicylic acid color reagent. The test tubes were incubated in a boiling water bath for 5 min, then cooled to room temperature. The reaction mixture was then diluted by adding 1.5 mL distilled water, and absorbance was measured at 540 nm using a microplate reader (SpectraMax, USA) by adding 200 µL in 96-well plates. The α-amylase inhibitory activity was expressed as % inhibition and also the concentrations of extract/ Acarbose resulting in 50% inhibition of enzyme activity (IC50) were determined.
In vitro α-glucosidase inhibitory activity of the extract: The assay was evaluated using the modified procedure of Li, et al. 2005 [25], and Akinwunmi and Ayoola, 2018 [24]. Alpha-glucosidase from Saccharomyces cerevisiae was purchased from Sigma. A volume of 50 µL of extract or standard drug (acarbose) and 100 µL of 0.1 M phosphate buffer (pH 6.9) containing α-glucosidase solution (1.0 U/mL) were incubated in 96-well plates at 25 °C for 10 min. After pre-incubation, 50 µL of 1 mM p-nitrophenyl-glucopyranoside solution in 0.1 M phosphate buffer (pH 6.9) was added to each well. The reaction mixtures were incubated at 25 °C for 20 min and stopped by adding 200 µL of 1M Na2CO3. The absorbance readings were recorded by a micro-plate reader at 405 nm and compared to a control which had 50 µL of buffer solution in place of the extract/acarbose. The α-glucosidase inhibitory activity was expressed as % inhibition and also the concentrations of extract/acarbose resulting in 50% inhibition of enzyme activity (IC50) were determined.
Antihyperglycaemic effect of the extract on glucose-induced hyperglycaemic rats: Hyperglycaemia was induced in groups of 6 rats each that were for fasted 18 hours by oral administration of 10 g/kg of glucose. After 0.5 hours (time point 0) of glucose administration, rats with blood glucose levels ≥7.0 mmol/L (126 mg/dL) were considered hyperglycaemic and given (p.o.) vehicle (Tween 80 (1%) in distilled water) (negative control) extract (100 200 and 400 mg/kg) separately and 5.0 mg/kg glibenclamide (positive control). At 0.00, 0.50, 1.00, 2.00, and 4.00 hours, a drop of blood from each rat’s caudal vein was placed onto a glucometer strip inserted into the glucometer. The percentage decrease in blood glucose levels at these time points was calculated and compared to the negative and positive controls [24,26].
Antihyperglycaemic effect of the extract on streptozotocin-induced diabetic rats: Diabetes was induced in overnight fasted rats by intraperitoneal injection with freshly prepared, 65 mg/kg streptozotocin (STZ) solution in 0.1 M sodium citrate buffer (pH 4.5). The blood glucose levels of the rats were observed after 72 hours of induction and they were left for 5 days afterwards. Rats with fasting blood sugar (FBS ≥ 11.0 mmol/l) were considered diabetic and separately divided into 4 groups of 8 rats viz; negative control that was orally given 1% Tween 80 in distilled water, test groups that received, 100 and 200 mg/kg (doses with the highest activity from the glucose-induced hyperglycaemic experiment); the positive control group that was administered with glibenclamide (5 mg/kg). Each group was treated daily accordingly for 14 days while blood glucose levels were monitored on days 1, 4, 7, 10, and 14 also the percentage of blood glucose reduction was determined and compared with that of the control [27,28]. All animal experiments were made to conform to the guide for the care and use of laboratory animals published by the national academies press [29].
Gas chromatographic – mass spectroscopy of the leaf extract
The GC-MS analysis of the leaf extract of C. zenkeri was carried out at the Nigerian Institute of Medical Research (NIMR), Yaba, Lagos State, Nigeria. The analysis was performed using an Agilent 5977B GC/MSD system coupled with Agilent 8860 auto-sampler, a Gas Chromatograph interfaced to a Mass Spectrometer (GC-MS) equipped with an Elite-5MS (5% diphenyl/95% dimethyl polysiloxane) fused a capillary column (30 × 0.25 μm ID × 0.25 μm df). For GC-MS detection, an electron ionization system was operated in electron impact mode with an ionization energy of 70 eV. Helium gas (99.999%) was used as a carrier gas at a constant flow rate of 1 ml/min, and an injection volume of 1 μL was employed (a split ratio of 10:1). Five (5) point serial dilution calibration standards (1.25, 2.5, 5.0, 10.0 ppm) were prepared from the stock solution of 40 ppm and used to calibrate the GC-MS.
The injector temperature was maintained at 300 °C, the ion-source temperature was 250 °C, and the oven temperature was programmed from 100 °C (isothermal for 0.5 min), with an increase of 20 °C/min to 280 °C (2.5 min), Mass spectra were taken at 70 eV; a scanning interval of 0.5 s and fragments from 45 to 450 Da. The solvent delay was 0 to 3 min, and the total GC/MS running time was 21.33 min. The data solution software supplied was used to control the system and acquire the data. The separated constituents were passed to the detector which recorded the emergence of the constituents as peaks with a retention time. The percentage compositions of the compound in the entire sample were computed from the peak areas automatically generated by the machine [10,30]. The results were recorded as retention time against percentage composition in the original sample.
Interpretation of mass spectrum GC-MS was conducted using the database of the National Institute Standard and Technology (NIST) having more than 62,000 patterns and the National Centre for Biotechnology Information. The spectrum of the unknown components was compared with the spectrum of known components stored in the NIST library. The data generated was tabulated to reflect the molecular formula, molecular weight, and peak area of each component identified by its retention time [10,30].
Statistical analysis
Data were represented as mean ± SEM for the number (n) of animals per group. Analysis of variance (ANOVA) then Student Newman Keul’s test was used to obtain the significant difference for all determinations. P < 0.050 was taken to be significant statistically.
Acute effect of Celtis zenkeri leaf extract in normal rats
In the acute toxicity test, single administration of 5000 mg/kg methanolic leaf extract of Celtis zenkeri to healthy rats did not cause any observable toxicity in the animals when observed daily for 14 days. None of the rats died and there were no observable changes in the behaviour of the rats with respect to breathing. Also, no negative cutaneous effect, sensory and nervous system responses, or gastrointestinal effect was observed. The median lethal dose, LD50 of the leaf extract was therefore greater than 5000 mg/kg which indicated its safety and that the dose range of 100 to 1000 mg/kg used in the study was therefore non-toxic.
Sub-acute effect of C. zenkeri leaf extract on blood glucose level in normal rats
There was no significant decrease in the blood glucose levels of normal rats that were given distilled water for the 21 days of the study which justified the neutrality of water. The extract at 100 mg/kg gave a 17% hypoglycaemic effect that was significantly higher than water on day 21 which indicated that prolonged administration of the extract at this dose may cause hypoglycaemia in normal subjects. In addition, increasing the dose of the extract to 500 and 1000 mg/kg elicited a significantly higher decrease in the blood glucose levels of the animals compared to the negative control on days 7-21 which called for caution in its use by normal subjects because it may precipitate hypoglycaemia (Table 1).
| Table 1: Effect of C. zenkeri leaf extract on blood glucose level of normal rats. | ||||
| Dose of Extract (mg/kg) | Day 1 | Day 7 | Day 14 | Day 21 |
| DW | 100 | 97.74 ± 1.32b | 96.57 ± 1.67b | 98.08 ± 1.95c |
| CZLE (250) | 100 | 91.46 ± 2.69b 6.43.46% |
88.66 ± 2.66b 9.19% |
81.50 ± 3.38b 16.90% |
| CZLE (500) | 100 | 84.86 ± 2.45a 13.18% |
79.49 ± 3.13a 17.69% |
75.63 ± 2.87b 22.89% |
| CZLE (1000) | 100 | 84.82 ± 5.12a 13.22% |
74.46 ± 6.46a 22.90% |
67.58 ± 6.95a 31.10% |
| Data show the mean ± SEM blood glucose levels at the different time points (Tt) expressed as percentages of the level at day 1, percentage reductions in the blood glucose levels relative to negative control for each time point, N = 8. Values with similar superscripts are comparable (p > 0.05). One-way analysis of variance followed by the Student Newman-Keuls’ post-hoc test). DW: Distilled Water; CZLE (250, 500, 1000): Celtis zenkeri methanolic leaf extract. | ||||
Sub-acute effect of the leaf extract of C. zenkeri on haematological parameters
Toxicity of plant extracts in living systems as well as the health status of animals can be assessed by monitoring haematological components of human/animals blood samples, such as red blood cells, white blood cells or leucocytes, mean corpuscular volume, mean corpuscular haemoglobin and mean corpuscular haemoglobin concentration. Twenty-one days of daily administration of 250, 500, and 1000 mg/kg of the leaf extract of C. zenkeri to normal rats in this study elicited a significant (p > 0.05) increase in the RBC level of the animal blood which suggested that the extract had anti-anaemic and immunomodulatory effects of the on the rats’ blood [31]. Senecio biafrae, Xylopia aethiopica, Xylopia aethiopica, and Spondia mombin extract had similarly been reported to increase the RBC level of normal rats [18]. The extract did not affect other haematological parameters of the animal blood when compared to the control which further confirmed its safety (Table 2).
| Table 2: Effect of extract on haematological parameters of normal rats. | ||||
| Blood Parameters | D W | CZLE (250. mg/kg) |
CZLE (500 mg/kg) |
CZLE (1000 mg/kg) |
| PCV (%) | 41.4 ± 0.11a | 44.0 ± 0.22b | 45.2 ± 0.71b | 46.3 ± 0.63b |
| HGB (g/dL) | 13.0 ± 0.10a | 12.86 ± 0.12a | 13.3 ± 0.31a | 13.6 ± 0.41a |
| RBC (106 /µL) | 6.67 ± 0.53a | 7.20 ± 0.34a | 7.12 ± 0.33a | 7.20 ± 0.67a |
| WBC (106/µL | 4.6 ± 0.23a | 4.80 ± 0.72a | 5.20 ± 0.82a | 4.40 ± 0.40a |
| MCV (fl) | 65.7 ± 0.14a | 62.0 ± 0.52a | 60.3 ± 0.87a | 61.6 ± 0.62a |
| MCH (pg) | 18.5 ± 0.11a | 20.7 ± 0.28a | 20.1 ± 0.23a | 20.5 ± 0.80a |
| MCHC (g/dL) | 32.25 ± 0.21a | 33.3 ± 0.26a | 33.3 ± 0.18a | 33.3 ± 0.74a |
| Data show the mean ± SEM haematological parameters at the different doses, n = 8. Results having separate superscripts within a row are significantly different (p < 0.050), while those that are alike are comparable (p > 0.050): One-way variance analysis (ANOVA) followed by Student-Newman-Keul’s test. DW: Distilled Water; CZLE (250, 500, 1000): Celtis zenkeri leaf extract. PCV: Parked Cell Volume; WBC: White Blood Corpuscles, RBC: Red Blood Corpuscles, HGB: Haemoglobin, MCV: Mean Corpuscular Volume, MCH: Mean Corpuscular Haemoglobin; MCHC: Mean Corpuscular Haemoglobin Concentration. |
||||
Effect of the leaf extract of Celtis zenkeri on biochemical parameters
In the investigation of the effect of Celtis zenkeri on the biochemical components of blood samples of animals after treatment for 21 days, the extract did not show any significant effect on aspartate transaminase (AST), alanine transaminase (ALT), creatinine and alkaline phosphatase levels when compared to distilled water (negative control) (Table 3). This implied that the extract did not have any toxic effect on the vital organs of the rats such as the heart, liver, and kidney. Significant elevation of AST and ALT levels would indicate liver damage while a high value of creatinine would indicate kidney damaging effect of the extract. However, a significant increase in cholesterol level compared to negative control that was observed at 500 and 1000 mg/kg of the extract indicated a possible hyperlipidemic effect of the extract at high doses (Table 3).
| Table 3: Effect of extract on biochemical parameters of normal rats. | ||||
| Biochemical Parameters | DW | CZLE (250 mg/kg) |
CZLE (500 mg/kg) |
CZLE (1000 mg/kg) |
| AST(µ/L) | 20 ± 0.12a | 22 ± 0.23a | 21 ± 0.34a | 19 ± 0.02a |
| ALT(µ/L) | 21 ± 0.02a | 19 ± 0.04a | 20.8 ± 0.02a | 22.4 ± 0.04a |
| CREA(mg/dL) | 1.2 ± 0.11a | 1.0 ± 0.07a | 1.4 ± 0.03a | 1.3 ± 0.01a |
| CHOL(mg/dL) | 55 ± 0.01a | 58 ± 0.22a | 64 ± 0.13b | 65 ± 0.05b |
| ALP(IU/L) | 58 ± 0.04a | 57 ± 0.02a | 55 ± 0.01a | 56 ± 0.06a |
| Data show the mean ± SEM biochemical parameters at different doses, n = 8. Results having separate superscripts within rows are significantly different (p < 0.050), while those that are alike are comparable (p > 0.05). DW: Distilled Water; CZLE (250, 500, 1000): Extract of Celtis zenkeri, AST: Aspartate Transaminase; ALT: Alanine Transaminase; CREA: Creatinine; CHOL: Cholesterol; ALP: Alkaline Phosphatase. |
||||
Antidiabetic studies of Celtis zenkeri leaf extract
Alpha amylase and α-glucosidase repress the intestinal enzymes which convert polysaccharides to monosaccharides. This causes delayed assimilation of carbohydrates after a meal due to inadequate polysaccharide absorption from the gastrointestinal tract. This lowers the risk of postprandial hyperglycemia in patients with insufficient pancreatic-cell reserves [32]. However, their side effects such as flatulence and other gastrointestinal problems necessitated a search for new α-amylase and α-glucosidase inhibitors of natural origin. The α-amylase inhibitory effect of C. zenkeri leaf extract in this study decreased with an increase in concentration while that of acarbose increased with concentration (Table 4). The extract was significantly more active than the positive control from 62.5-250 µg/mL while acarbose was significantly more active than the extract at 1000 µg/mL.
| Table 4:In-vitro α-amylase inhibitory activity of Celtis zenkeri leaf extract. | ||
| Concentration of the extract (µg/mL) |
Percentage α-amylase Inhibition (%) | |
| Celtis zenkeri | Acarbose | |
| 62.5 | 64.80 ± 1.67b | 17.66 ± 0.23a |
| 125 | 55.43 ± 1.43b | 22.27 ± 1.33a |
| 250 | 52.96 ± 1.45b | 32.12 ± 1.23a |
| 500 | 47.04 ± 0.69a | 49.74 ± 0.25a |
| 1000 | 40.13 ± 1.09a | 56.99 ± 0.44b |
| Data show the mean ± SEM (n = 6). Values with different superscripts within columns are significantly different (p < 0.05), one-way analysis of variance followed by the Student–Newman–Keuls’ test). | ||
Contrary to the α-amylase inhibitory activity of C. zenkeri leaf extract, its α-glucosidase inhibitory effect increased with concentration from 62.5-1000 µg/mL similar to that of acarbose (Table 5). The positive control however elicited a significant ability to inhibit carbohydrate breakdown than the extract at all tested concentrations. The combined α-amylase and α-glucosidase inhibitory effects of C. zenkeri leaf extract showed its potential for use in the management of diabetes. Similar activities have been reported for Morinda lucida, Musa paradisiacal, and Nuxia oppositifolia [33-35].
| Table 5: Alpha-glucosidase inhibitory effect ofCeltis zenkeri leaf extract. | ||
| Concentration of extract (µg/mL) | Average percentage inhibition (%) | |
| Celtis zenkeri | Acarbose | |
| 62.5 | 57.69 ± 3.58a | 74.12 ± 0.32b |
| 125 | 61.85 ± 4.09a | 76.81 ± 1.20b |
| 250 | 76.76 ± 5.49a | 88.21 ± 1.24b |
| 500 | 86.82 ± 3.85a | 94.73 ± 1.25b |
| 1000 | 88.71 ± 0.31a | 96.48 ± 0.62b |
| Data show the mean ± SEM (n = 6). Values with different superscripts within columns are significantly different (p < 0.05), one-way analysis of variance followed by the Student–Newman–Keuls’ test). | ||
The results of the Oral Glucose Tolerance Test (OGTT) model when insulinotropic drugs such as glimepiride, glibenclamide, and others are used as positive controls, have been reported to resemble type 2 diabetes state in humans [36]. Such have been reportedly used in the investigations of medicinal plants for their anti-hyperglycaemic activities [37,38] and hence used in this study. The negative control group of rats that were given 10 g/kg glucose elicited a significant (p < 0.050) decrease in blood glucose level till the fourth hour due to the homeostatic regulatory mechanism in normal animals (Table 6) [37,39]. The leaf extract of C. zenkeri at 100 mg/kg lacked an antihyperglycaemic effect at 0.5-2 h but gave 19% activity at 4 h which was significantly lower (p > 0.05) than 45, 47, and 39% given by 200, 400 mg/kg and glibenclamide (5 mg/kg), respectively. Both 200 and 400 mg/kg of the extract gave comparable (p < 0.05) effect that was significantly better than 100 mg/kg and similar to the profile of the activity of glibenclamide at 0.5-4 h. This suggested that the extract may have similar minor extrapancreatic and major insulin-stimulating mechanisms of action of glibenclamide [40]. Also, the extract at 200 and 400 mg/kg was comparable in activity to glibenclamide at all time points except 200 mg/kg which showed a more significant effect at 2 h.
| Table 6: Dose-related antihyperglycaemic effect of Celtis zenkeri leaf extract. | ||||||
| Extract/Drug (mg/kg) |
Blood glucose levels as percentages of To (% reduction in blood glucose relative to negative control at To) | |||||
| 0h | 0.5 h | 1 h | 2 h | 4 h | ||
| DW | 100.00 | 83.79 ± 3.81a | 85.89 ± 0.50b | 76.45 ± 1.71c | 74.18 ± 1.97c | |
| CZLE (100) | 100.00 | 93.17 ± 3.16b (-11.19%) |
88.54 ± 3.92b (-3.09%) |
79.03 ± 6.61c (-3.37%) |
60.11 ± 3.33b (18.97%) |
|
| CZLE (200) | 100.00 | 87.99 ± 1.79a (-5.01%) |
68.49 ± 4.46a (20.26%) |
50.01 ± 3.82b (34.58%) |
40.90 ± 1.03a (44.86%) |
|
| CZLE (400) | 100.00 | 86.92 ± 1.94a (-3.74%) |
65.87 ± 2.64a (23.31%) |
58.19 ± 2.60a (23.88%) |
38.98 ± 2.91a (47.45%) |
|
| GLI (5) | 100.00 | 75.64 ± 6.73a (9.73%) | 70.68 ± 6.86a (17.71%) | 58.32 ± 6.44a (23.72%) | 45.27 ± 6.88a (38.97%) | |
| Data show the mean ± SEM blood glucose levels at the different time points expressed as a percentage of levels at 0 h, n = 6. Values with different superscripts within the column are significantly different (p < 0.05), while values with similar superscripts are comparable (p > 0.05): one-way analysis of variance (ANOVA) followed by Student-Newman-Keul’s test. GLU (10 g/kg): Glucose 10 g/kg; CZLE: Celtis Zenkeri Leaf Extract, GLI: Glibenclamide (Positive Control, 5 mg/kg). | ||||||
In an attempt to further establish the antidiabetic activity of C. zenkeri leaf extract in this study, the two most antihyperglycaemic doses in the glucose-loaded experiment, 100 and 200 mg/kg (Table 4) were used in streptozotocin-induced diabetic rats. The negative group of diabetic rats that were given distilled water maintained a high diabetic state throughout the study period showing that diabetes induced by the administered streptozotocin was permanent (Figure 1).
Figure 1: Effect of C. zenkeri leaf extract in streptozotocin-induced diabetic rats.
A significantly time-dependent antidiabetic activity of 14, 56, 78, and 83% on days 4, 7, 10 and 14, respectively was elicited by the positive control drug (glibenclamide 5 mg/kg) which was due to insulin stimulating action of the drug on the remaining pancreatic β-cells of the diabetic rats. The extract of C. zenkeri at 200 mg/kg and glibenclamide (5 mg/kg) showed early onset of action with a comparable (p > 0.05) antidiabetic effect on day 4 that was significantly (p < 0.05) higher than that of 100 mg/kg dose on the same day. On day 7 however, the 100 mg/kg dose was significantly more active than 200 mg/kg of the extract and glibenclamide while it is 100 and 200 mg/kg were comparable in activity with the positive control on days 10 and 14 (Figure 1). This result showed the efficacy of the extract in the reduction of hyperglycaemia in diabetic condition and further confirmed the insulin stimulating effect of the extract that was suggested by the Oral glucose tolerant test Figure 2.
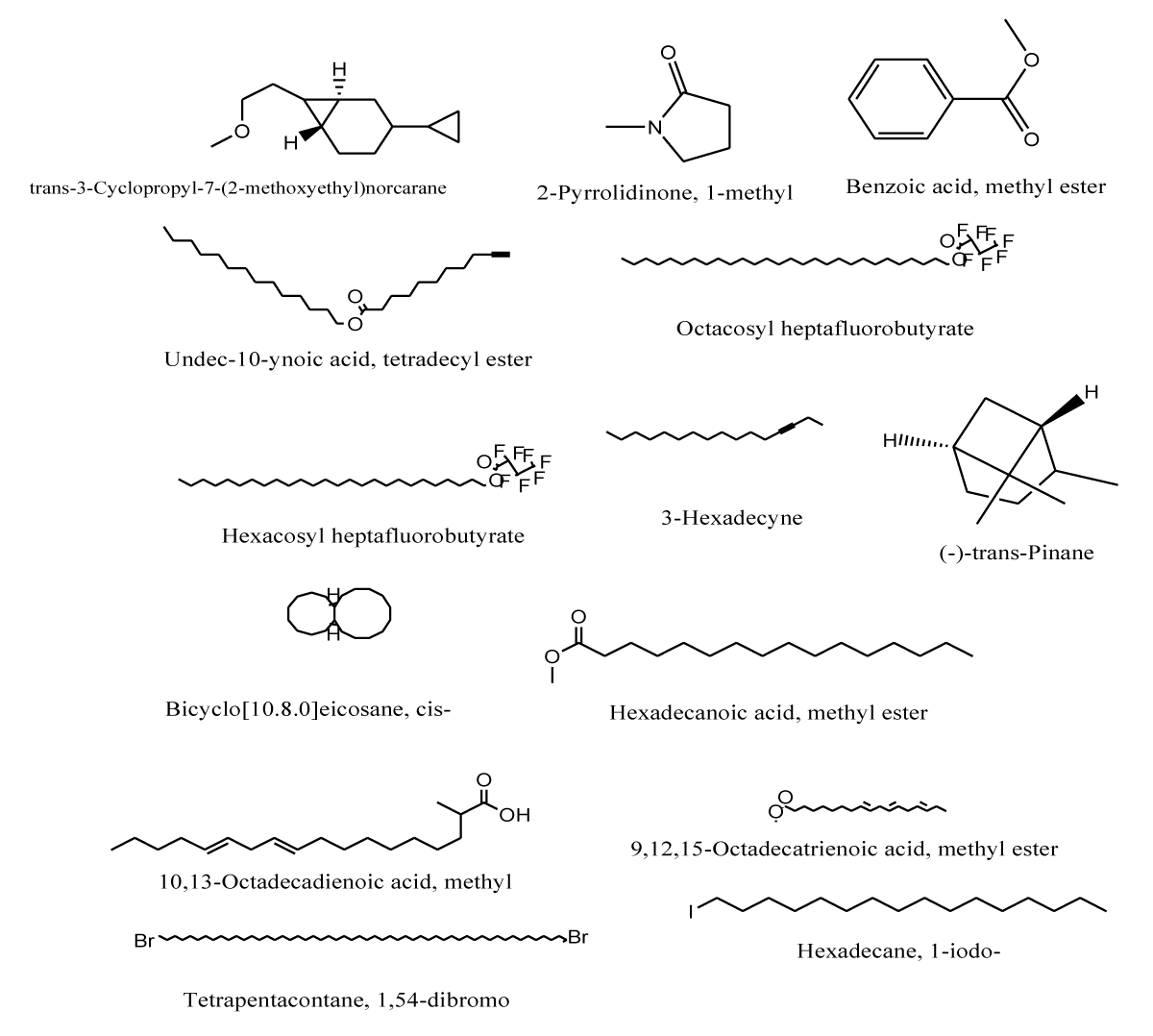
Figure 2: Chemical structures of the identified and characterised compounds from the methanolic leaf extract of Celtis zenkeri.
Gas chromatographic – mass spectroscopy of the leaf extract
In the GC-MS analysis of C. zenkeri leaf extract, 14 compounds representing 14 peaks were characterised from about 20 peaks that were obtained from the entire spectrum which indicated that the extract had many components with the first peak having an Rt of 4.02 while the last component was 16.48. The most abundant component of the extract was the one with Rt 13.31 (22.64%) while other abundant peaks were those with Rt 14.74, 14.83, and 12.58 with peak areas of 15.25, 17.67, and 11.49%, respectively. Other prominent peaks (2% - 8%) are 14.68 (8.04), 16.45 (4.74), 12.95 (6.32), 11.49 (3.18), 12.79 (2.40), while those of 4.70(1.25), 4.08(1.19), 9.63(0.4), 4.02 (0.38), 8.15 (0.32), (0% - 1.5%) were traces (Table 7).
| Table 7: Gas-Chromatographic analysis of the leaf extract of Celtis zenkeri. | ||||||
| SN | NAME | MW | FORMULA | CAS NO. | RT (Mins) | PEAK AREA |
| 1 | trans-3-Cyclopropyl-7-(2-methoxyethyl) norcarane | 194.31 | C13H22O | 1000223-15-8 | 4.020 | 0.38 |
| 2 | 2-Pyrrolidinone, 1-methyl- | 99.13 | C5H9NO | 000872-50-4 | 4.071 | 1.19 |
| 3 | Benzoic acid, methyl ester | 136.1479 | C8H8O | 000093-58-3 | 4.707 | 1.25 |
| 4 | Undec-10-ynoic acid, tetradecyl ester | 378.6 | C25H46O2 | 1000406-16-7 | 8.151 | 0.32 |
| 5 | Octacosyl heptafluorobutyrate | 606.7826 | C32H57F7O· | 1000351-83-6 | 9.633 | 0.4 |
| 6 | Hexacosyl heptafluorobutyrate | 578.7294 | C30H53F7O | 1000351-83-3 | 11.493 | 3.18 |
| 7 | 3-Hexadecyne | 222.4094 | C16H30 | 061886-62-2 | 12.580 | 11.49 |
| 8 | (-)-trans-Pinane | 138.2499. | C10H18 | 033626-25-4 | 12.798 | 2.40 |
| 9 | Bicyclo[10.8.0]eicosane, cis- | 278.516 | C20H38 | 1000155-82-2 | 12.952 | 6.32 |
| 10 | Hexadecanoic acid, methyl ester | 270.4507 | C17H34O | 000112-39-0 | 13.313 | 22.64 |
| 11 | 10,13-Octadecadienoic acid, methyl | 294.5 | C19H34O2 | 056554-62-2 | 14.686 | 8.04 |
| 12 | 9,12,15-Octadecatrienoic acid, methyl ester | 292.4562 | C19H32O | 000301-00-8 | 14.737 | 15.25 |
| 13 | Tetrapentacontane, 1,54-dibromo | C24H108Br2 | 1000156-09-4 70 | 14.829 | 17.67 | |
| 14 | Hexadecane, 1-iodo- | 352.34 | C16H33I | 000544-77-4 90 | 16.488 | 4.74 |
The most abundant peak (13.31, 22.64%) was characterised as Hexadecanoic acid, methyl ester with molecular formula C17H34O and molecular weight 270.4507. Also, the relatively abundant peaks (Rt 14.829, 14.737, and 12.580 with peak areas 15.25, 17.67, and 11.49 respectively of the extract were characterised as Tetrapentacontane1,54-dibromo, 9,12,15-Octadecatrienoic acid, methyl ester, and 3-Hexadecyne respectively. The chloroform leaf extract of Ximenia americana had been shown by GC-MS analysis to contain octadecatrienoic acid and n-Hexadecanoic acid while its aqueous extract contained 9, 12-Octadecadinoic acid which was reported for its α-amylase and α-glucosidase inhibitory activities [41]. This suggested that these compounds that were similarly obtained in C. zenkeri extract would contribute to its observed antidiabetic activities. Studies have reported that both natural monoterpenes and their synthetic derivatives have a vast array of pharmacological actions including anti-diabetic, hypocholesterolemic, antioxidant, antibacterial, anti-inflammatory, anti-cancer, antihistaminic, and analgesic actions [42]. Some monoterpenes which can be identified in C. zenkeri might have been responsible for its observed antidiabetic activities [43-45].
Histopathological studies of the effect of leaf extract of C. zenkeri
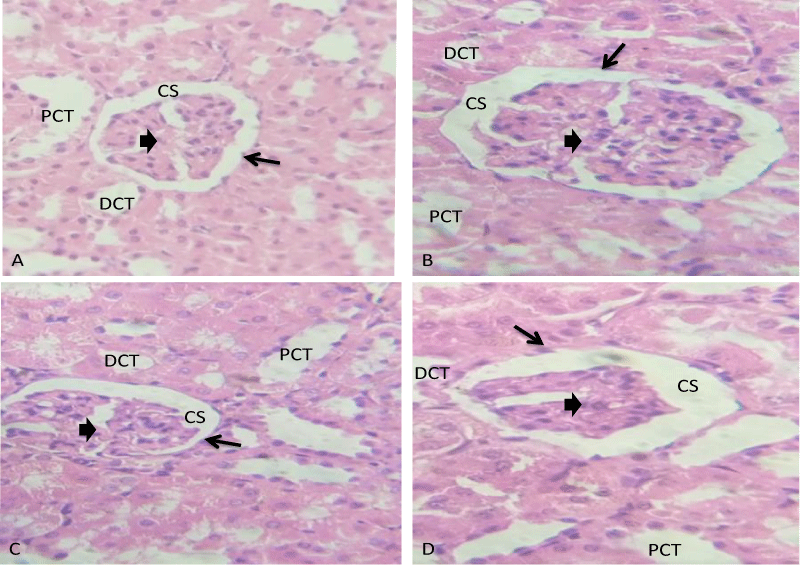
The photomicrograph of the histology of renal tissue of the control group of rats in this study showed normal histoarchitecture. The glomerulus (black thick arrow), capsular space (CS), epithelia cells (black thin arrow) and the proximal convulated tubules (PCT) and Distal convulated tubules) DCT) appeared normal. The extract at 250 and 500 mg/kg showed no significant morphological changes in the tissue. The glomerulus (black thick arrow), epithelial cells (black thin arrow), capsular space (CS), and the proximal and distal convulated tubules (PCT and DCT) were well organized without any loss of function. However, there was distorted glomerulus (black thick arrow), constricted distal convulated tubules (DCT), capsular space (CS) with dilated proximal convoluted tubules (PCT) in the renal tissue of the rats treated with 1000 mg/kg of the extract. This indicated possible adverse effect of the extract on the kidney at this dose (Plate 1).
Plate 1: Photomicrographs of the histology of renal tissue A: Control group, B-D: Treatment groups with 250, 500 and 1000 mg/kg of Celtis zenkeri leaf extract, respectively. H/EX400.
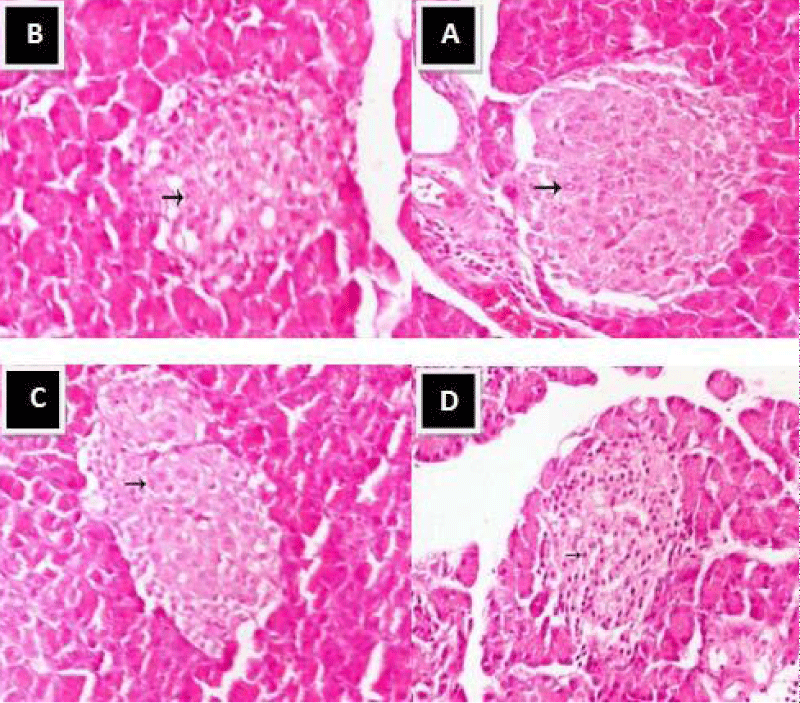
Histopathology of the pancreas of normal control rats (A) showed normal islets of Langerhans with pale rounded and ovoid β-cells in the center (arrow), embedded in exocrine portion of pancreas. In the pancreas of rats treated with 250 mg/kg of extract (B), the islets of Langerhans remained normal sized but some degeneration of the β cell in the center were noticed (arrow). There were normal islets of Langerhans with its normal pale large round to ovoid shaped containing cells (arrow) that embedded in exocrine portion of pancreas in the pancreas of the rats treated with 500 mg/kg of CZ. However, the extract at 1000 mg/kg caused shrinkage of islets of Langerhans with degeneration and necrosis of components cells. This suggested that the extract was not safe for use at elevated doses (Plate 2).
Plate 2: Photomicrograph of the histology of pancreatic tissue A: Control group, B-D: Treatment groups with 250, 500 and 1000 mg/kg of Celtis zenkeri extract, respectively. (H&E, x400).
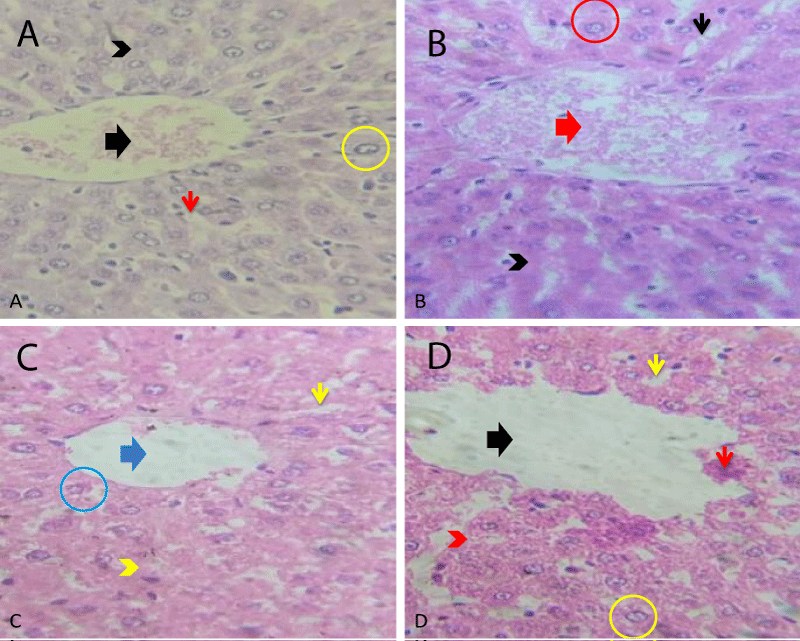
In the liver tissue histology of the control group of rats, the central vein (black thick arrow), hepatocytes (yellow circle), sinusoids housing the kupfer cells (red thin arrow) and the hepatic plate (black arrow head) were well defined. There were no severe morphological changes in the hepatic plate (black arrow head), hepatocytes (red circle) of rats treated with 250 and 500 mg/kg. Slight congestion of the central vein (red thick arrow) and sinusoids (black thin arrow), mild morphological changes, irregularity of the hepatic plate (yellow arrow head) and constricted sinusoid (yellow thin arrow) with a clear central vein (blue thick arrow) were observed in the group of rats treated with 1000 mg/kg of the extract. This showed possible hepatotoxic effect of the extract at high doses (Plate 3).
Plate 3: Photomicrographs of the histology of liver tissue A: Control group, B-D: Treatment groups with 250, 500 and 1000 mg/kg of C. zenkeri extract, respectively. H/E X400.
The results of both the in vitro and in vivo antidiabetic activities of the leaf extract of Celtis zenkeri as well as its toxicological studies obtained in this work confirmed its potency and safety and therefore justified its folkloric antidiabetic usage.
The authors wish to appreciate the assistance of Mr. I. I. Ogunlowo for his efforts in collecting the plant that was used in this study.
- Trease GE, Evans WC. Pharmacognosy. Fifteenth edition, Edinburgh, London, New York. 2002; 1-2.
- Lai PK, Roy J. Antimicrobial and chemopreventive properties of herbs and spices. Curr Med Chem. 2004 Jun;11(11):1451-60. doi: 10.2174/0929867043365107. PMID: 15180577.
- Tapsell LC, Hemphill I, Cobiac L, Patch CS, Sullivan DR, Fenech M, Roodenrys S, Keogh JB, Clifton PM, Williams PG, Fazio VA, Inge KE. Health benefits of herbs and spices: the past, the present, the future. Med J Aust. 2006 Aug 21;185(S4):S1-S24. doi: 10.5694/j.1326-5377.2006.tb00548.x. PMID: 17022438.
- Sofowora A. Medicinal Plants and Traditional Medicine I. Africa. 3rd edition, Spectrum Books Ltd., Ibadan, 2008; 117-133.
- Zargari A. Medicinal Plants. Tehran University Press. 1992; 889.
- Essien C, Oteng-Amoako AA. Celtis zenkeri Engl. Record from PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands 2012.
- Adjanohoun E, Aké Assi L, Adjakidjè V. Contribution to ethnobotanical and floristic studies in the People's Republic of Congo, 2019. Médecine d'Afrique Noire, 65(6): 344-354.
- Lowe HI, Khan MA, Geevarghese S. The West African Nettle Tree (Celtis zenkeri) in the Spotlight: A Review of Its Traditional Uses. Phytochemistry and Pharmacology. 2021; 10(7): 1442.
- Okoye FBC, Akunneh-Wariso C, Ihedioha NJ. Phytochemical and antioxidant analysis of the stem bark extract of Celtis zenkeri, 2021, International Journal of Plant and Animal Sciences. 9(1): 1-9.
- Okpala E, Onocha PA, Ali M. Zenkeramide: a new iso-benzofuranone propanamide and urease inhibitory constituents of Celtis zenkeri Engl stem bark (Ulmaceae). Trends in Phytochemical Research. 202;; 6(2): 137-144.
- Adeniji AJ, Adewale OB, Adekeye BT, Adekoya AO, Adeniji OB, Ajayi AM. In vitro cytotoxicity and apoptotic effect of Celtis zenkeri leaf extract against MCF-7 human breast cancer cell line. Journal of Medicinal Plants for Economic Development. 2020; 4(1): 1-9.
- Akinpelu OA, Omololu-Aso J, OkohA I. Cytotoxicity and apoptosis-inducing effect of Celtis zenkeri leaves extract against HeLa cervical cancer cell lines. 2021. Journal of Complementary and Integrative Medicine. 18(2): 1-9.
- Folarin RO, Alade GO, Olorunsogo OO, Oridupa OA. Antidiabetic and hypolipidemic effect of methanol extract of Celtis zenkeri leaves in streptozotocin-induced diabetic rats, South African Journal of Botany. 2021; 140: 206-211.
- Tchoumbougnang F, Ekué MRM, Mapongmetsem PM, Wouatsa NA. Antifungal activity of the extracts of bark from Celtis zenkeri. International Journal of Microbiology and Mycology. 2019; 8(4): 396-402.
- Oyedeji AO, Adeyemi OA, Aremu OJ, Afolayan AJ. Evaluation of Phytochemical Composition, Antioxidant and Antimicrobial Activities of Celtis zenkeri. Journal of Medicinal Plants Research. 2020; 14(2): 63.
- Okpala EO, Onocha P, Ali MS, Ur-Rehmenc SZ, Lateef M. Antioxidant activity of phytol dominated stem bark and leaf essential oils of Celtis zenkeri Engl. Natural Product Research. 2021; 6(2): 137-144
- OECD. The OECD guidelines for the testing of chemicals: 423. Acute Oral Toxicity – Acute Toxic Class Method, 2001.
- Bello OI, Ayoola MD, Obembe O, Akinwunmi KF. Antidiabetic and Toxicity Studies of the Extract of Four Nigerian Medicinal Plants. European Journal of Medicinal Plants. 2020; 33(11):32-45.
- OECD. The OECD guidelines for the testing of chemicals: 407. Repeated dose 28-day oral toxicity study in rodents, 2008.
- Hemant S, Tun-Abraham ME, McAlister V. Bidirectional graft-host hematological traffic in liver transplantation. 2019; Hepatobiliary Surgery and Nutrition. 8: 253.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18(6):499-502. PMID: 4337382.
- Joseph BF, Reginald Edward Silverton. Introduction to medical laboratory technology (Butterworth-Heinemann). 2014.
- Bahman N, Leyla A, Hamidreza I. Alpha-amylase inhibitory activities of six Salvia spp. Iranian J. Pharm. Res. 2008; 7:297-303.
- Akinwumi KF, Ayoola MD. Antihyperglycaemic, anti-inflammatory and antioxidant activities of Xylopia aethiopica and Citrullus lanatus seeds extracts. Ife Journal of Science. 2018; 20(2):207-218.
- Li T, Zhang XD, Song YW. A microplate-based screening method for alpha-glucosidase inhibitors. Chinese Chin J Clin Pharmaco Ther. 2005; 10:1128-1134.
- Ajileye AJ, Ayoola MD, Elujoba AA, Akinwunmi KF. Antihyperglycaemic and Antioxidant Activities of Sansevieria liberica as Justification for its Antidiabetic Claims. African Journal of Pharmacy and Pharmacology. 2020; 14(3):59-66.
- Ayoola MD, Balogun JO, Famuyiwa FG, Yeboah SO, Famuyiwa SO. Isolation and characterization of 2-hydroxy-3-[4-hydroxyphenyl]-2-propenoic acid and 4-bromophenol from anti-diabetic extract of the root bark of Uvaria afzelii. South African Journal of Botany. 2017; 112:527-532.
- Faloye KO, Ayoola MD, Amos-Tautau BM, Famuyiwa SO. Anti-diabetic activity of convallatoxin isolated from the root bark of Parquetina nigrescence (Afzel.) Bullock (Asclepidaceae), European Journal of Medicinal Plants. 2018; 25(4):1-9.
- Committee for the update of the guide for the care and use of laboratory animals, institute for laboratory animal research, division on earth and life studies, national research council of the national academies. Guide for the Care and Use of Laboratory Animals, 8th ed. The National Academies Press, Washington, DC. 2011.
- Sulaimon LA, Anise EO, Obuotor EM, Samuel AI, Moshood TA, Olajide M, Fatoke T. In vitro antidiabetic potentials, antioxidant activities and phytochemical profile of African black pepper (Piper guineense). Clinical Phytoscience. 2020; 6:1-13.
- Bashir L, Shittu OK, Rotimi AA, Olalekan IA, Kamooru AA, and Ossai PC. Effect of Methanol Extract of Telfairia occcidentalis on Haematological Parameters in Wister Rats. J. Med. Sci. 2015; 15(5):246-250.
- Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006 Aug;29(8):1963-72. doi: 10.2337/dc06-9912. PMID: 16873813.
- Kazeem MI, Adamson JO, Ogunwande IA. Modes of inhibition of α -amylase and α -glucosidase by aqueous extract of Morinda lucida Benth leaf. Biomed Res Int. 2013;2013:527570. doi: 10.1155/2013/527570. Epub 2013 Dec 24. PMID: 24455701; PMCID: PMC3884628.
- Shodehinde SA, Ademiluyi AO, Oboh G, Akindahunsi AA. Contribution of Musa paradisiaca in the inhibition of α-amylase, α-glucosidase and Angiotensin-I converting enzyme in streptozotocin induced rats. Life Sci. 2015 Jul 15;133:8-14. doi: 10.1016/j.lfs.2015.03.026. Epub 2015 Apr 25. PMID: 25921768.
- Alqahtani AS, Hidayathulla S, Rehman MT, ElGamal AA, Al-Massarani S, Razmovski-Naumovski V, Alqahtani MS, El Dib RA, AlAjmi MF. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomolecules. 2019 Dec 30;10(1):61. doi: 10.3390/biom10010061. PMID: 31905962; PMCID: PMC7022278.
- Verspohl EJ. Recommended testing in diabetes research. Planta Med. 2002 Jul;68(7):581-90. doi: 10.1055/s-2002-32894. PMID: 12142989.
- Adebajo AC, Ayoola MD, Odediran SA, Aladesanmi AJ, Schmidt TJ, Verspohl EJ. Evaluation of ethnomedical claim III: anti-hyperglycemic activities of Gongronema latifolium root and stem. J Diabetes. 2013 Sep;5(3):336-43. doi: 10.1111/1753-0407.12019. Epub 2013 May 28. PMID: 23217111.
- Adebajo AC, Ayoola MD, Obagbemi OR, Obuotor EM, Ogunsina MO, Verspohl EJ. Antihyperglycemic and Antioxidant Activities of Eugenia uniflora Leaf: Evaluation of Ethnomedicinal Claims IV, Ife Journal of Science and Technology. 2013b; 1(1):1-18.
- Adebajo AC, Iwalewa EO, Obuotor EM, Ibikunle GF, Omisore NO, Adewunmi CO, Obaparusi OO, Klaes M, Adetogun GE, Schmidt TJ, Verspohl EJ. Pharmacological properties of the extract and some isolated compounds of Clausena lansium stem bark: anti-trichomonal, antidiabetic, anti-inflammatory, hepatoprotective and antioxidant effects. J Ethnopharmacol. 2009 Feb 25;122(1):10-9. doi: 10.1016/j.jep.2008.11.015. Epub 2008 Nov 27. PMID: 19095054.
- Luzi L, Pozza G. Glibenclamide: an old drug with a novel mechanism of action? Acta Diabetol. 1997 Dec;34(4):239-44. doi: 10.1007/s005920050081. PMID: 9451465.
- Shettar AK, Sateesh MK, Kaliwal BB, Vedamurthy AB. In vitro antidiabetic activities and GC-MS phytochemical analysis of Ximenia americana extracts, South African Journal of Botany. 2017; 111:202-211.
- Al Kury LT, Abdoh A, Ikbariah K, Sadek B, Mahgoub M. In Vitro and In Vivo Antidiabetic Potential of Monoterpenoids: An Update. Molecules. 2021 Dec 29;27(1):182. doi: 10.3390/molecules27010182. PMID: 35011414; PMCID: PMC8746715.
- Tan XC, Chua KH, Ravishankar Ram M, Kuppusamy UR. Monoterpenes: Novel insights into their biological effects and roles on glucose uptake and lipid metabolism in 3T3-L1 adipocytes. Food Chem. 2016 Apr 1;196:242-50. doi: 10.1016/j.foodchem.2015.09.042. Epub 2015 Sep 12. PMID: 26593489.
- Guimarães AC, Meireles LM, Lemos MF, Guimarães MCC, Endringer DC, Fronza M, Scherer R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules. 2019 Jul 5;24(13):2471. doi: 10.3390/molecules24132471. PMID: 31284397; PMCID: PMC6651100.
- Kozioł A, Stryjewska A, Librowski T, Sałat K, Gaweł M, Moniczewski A, Lochyński S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev Med Chem. 2014;14(14):1156-68. doi: 10.2174/1389557514666141127145820. PMID: 25429661.