More Information
Submitted: February 29, 2024 | Approved: March 18, 2024 | Published: March 19, 2024
How to cite this article: Vaz CF, Mariano AF, Fracasso JAR, Ramos MVV,dos Santos L, et al. Evaluation of the Anti-inflammatory Activity of Equisetum arvense and Baccharis trimera Fractions. Arch Pharm Pharma Sci. 2024; 8: 003-008.
DOI: 10.29328/journal.apps.1001049
Copyright License: © 2024 Vaz CF, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and repro-duction in any medium, provided the original work is properly cited.
Keywords: E. arvense; B. trimera; Nutrition; Phytochemistry
Evaluation of the Anti-inflammatory Activity of Equisetum arvense and Baccharis trimera Fractions
Carolina Ferreira Vaz1# , Alan Fernandes Mariano1#
, Alan Fernandes Mariano1# , Júlia Amanda Rodrigues Fracasso2#
, Júlia Amanda Rodrigues Fracasso2# , Marcus Vinicius Vieitas Ramos3, Lucineia dos Santos4
, Marcus Vinicius Vieitas Ramos3, Lucineia dos Santos4 and Herbert Júnior Dias1*
and Herbert Júnior Dias1*
1Chemistry Center, Instituto Federal de Educação, Ciência e Tecnologia Goiano - Campus Urutaí, Urutaí – GO, Brazil
2Faculty of Dentistry, Universidade Estadual Paulista (UNESP), Araçatuba – SP, Brazil
3#Biology Center, Instituto Federal de Educação, Ciência e Tecnologia Goiano - Campus Urutaí, Urutaí – GO, Brazil
4#Faculty of Sciences and Letters, Universidade Estadual Paulista (UNESP), Assis – SP, Brazil
#The authors equally contribute to this work
*Address for Correspondence: Herbert Júnior Dias, Chemistry Center, Instituto Federal de Educação, Ciência e Tecnologia Goiano - Campus Urutaí, Urutaí – GO, Brazil, Email: [email protected]
Inflammation is a natural response of the body to defend itself against potential threats and can be reduced through physical activity, proper nutrition, and the use of herbal medicines, which are medicinal plants. In the study, we aim to examine the anti-inflammatory effects of the volatile and ethanolic fractions of two commonly used medicinal plants, Equisetum arvense, and Baccharis trimera. The essential oils were obtained by hydrodistillation of the fresh leaves of the plants, while the ethanolic extracts were obtained using classical methodologies. All fractions were tested for anti-inflammatory activity, evaluating their ability to stabilize the red blood cell membrane and inhibit the spreading, and phagocytosis by macrophages, at concentrations varying from 200 to 600 µg mL-1. The results of the experiments suggest that the ethanolic fraction of B. trimera shows promising results compared to the positive controls. Our investigations thus contribute to the specialized literature on the use of herbal medicines around nutrition, providing guidance for future studies on these fractions.
Inflammation is a response of the human body against various physiological and pathological processes, aimed at protecting the body against infections [1,2]. There are two types of inflammation: chronic and acute [3]. The use of commercial medications has been common in the treatment of inflammatory processes [4]. Another way to treat inflammation is through physical activity, as it promotes greater release of T regulatory cells, less secretion of immunoglobulins, and interferes with the th1/th2 balance, resulting in the production of th1 cells [5]. However, the use of herbal medicines and the ethnopharmacological knowledge of substances present in medicinal plants have gained prominence for their role as antagonists in the treatment of recurrent inflammatory diseases individuals’ daily lives [6,7].
Natural products, such as plant extracts, contain metabolites that can have beneficial effects on the body, such as inhibiting the formation of free radicals and modulating enzymes, known as special metabolites [8]. In folk medicine, many medicinal plants are already being used for various purposes, including supplementary, pharmacological, dietary applications, among others [9]. These fractions can be consumed in their natural form, as extracts, infusions, and other preparations. The special metabolites present in plants are produced by them for purposes such as pollination, controlling microbiological activity, defense, and attraction. These metabolites can be applied to humans for various purposes, such as treating or preventing inflammation [10]. Recent estimates from the literature suggest that over the last 40 years, approximately 23.5% of approved drugs or pharmaceuticals have been derived from medicinal plants [11]. Therefore, it is justifiable to propose more detailed studies on the biological potential of substances from Brazilian flora, as well as plants used in its traditional medicine.
Among the various cultivable plants in this region, medicinal plants with phytotherapeutic effects known in popular medicine as Equisetum arvense L. and Baccharis trimera have great biological potential that is still little explored for various applications [12]. E. arvense L is popularly known as “cavalinha” and is listed in the 1st edition of the Brazilian Pharmacopoeia, as well as on the National List of Medicinal Plants of Interest to the Unified Health System of Brazil (RENISUS). It is highly rich in minerals and is indicated by folk medicine for mild diuresis, swelling, remineralization, and inflammation [12]. “Carqueja” (B. trimera) is used to treat gastric and liver disorders, in addition to having antimicrobial properties and providing protection for the liver and stomach [13]. However, there are few studies on the anti-inflammatory activities of these plants and their fractions. Therefore, additional research is needed to explore the potential of these plants in inflammation, as the infusions or extracts of B. trimera and E. arvense have been used extensively by ethnopharmacology to increase the performance of physical practices. The purpose of this study is to extract polar and nonpolar fractions from E. arvense and B. trimera, and to test their anti-inflammatory potential.
Collection and identification of species
Intact leaves of E. arvense L. e B. trimera were collected from a native plant greenhouse, in the city of Orizona, GO, Brazil, with geographic coordinates 17°02’13.6”S 48°18’22.1”W. During the collection of plant samples, various parts of the species were obtained, including leaves and branches free from herbivory. All parts collected were sent as exsiccates for botanical classification of the plant species. The taxonomic classification was carried out by professor Dr. Marcus Vinícius Vieitas Ramos, and the exsiccates were stored in the herbarium of the Instituto Federal Goiano – Campus Urutaí- GO.
Obtaining natural product fractions
Leaves of B. trimera and E. arvense were used to extract volatile fractions, with 90 g and 174 g of fresh plant material samples used, respectively. This extraction was done through hydrodistillation in a Clevenger-type apparatus, adapted to a round-bottom flask, described in the literature and in subsequent works by the research group [14,15]. The fresh leaves were manually miniaturized, and a volume of 400 mL of distilled water was added to the flask, and the hydrodistillation process was carried out for 180 minutes. The organic fraction obtained was chemically dried with anhydrous magnesium sulfate and then filtered to remove the solid. To obtain the ethanolic extracts, freshly collected leaves (120 g in both cases) were dried in an oven, miniaturized, and placed in a 2 L flask [16]. A volume of 1200 mL of ethanol (P.A., 95%, Neon, São Paulo-SP, Brazil) was added to this mixture. It was manually stirred for 7 days and then filtered. The extract was evaporated at 40 °C under reduced pressure to dryness in a rotary evaporator (Fisatom 801 model, Fisatom, São Paulo-SP, Brazil). The remaining fraction was dried at room temperature in a desiccator. The fractions obtained were stored in a hermetically sealed amber flask and kept under refrigeration until used for biological analysis.
Evaluation of anti-inflammatory activities
To assess the anti-inflammatory effects of B. trimera and E. arvense, three experiments were conducted. Initially, for the evaluation of the human red blood cell (HRBC) membrane stabilization, the method proposed by Singh, et al. 2020 and Fracasso, et al. 2023 was employed, with some modifications [17,18]. The evaluation of the stabilization of the HRBC membrane was performed by creating a suitable biological medium to test the stability of the red blood cell membrane. In this test the evaluation of the in vitro anti-inflammatory activity was conducted with red cell homogenates (10%, w/v). Peripheral blood samples were obtained by the Laboratory of Clinical Analysis Bioanalysis, in the city of Cândido Mota (SP). The samples that were donated corresponded to the leftovers of whole human blood that would be discarded after carrying out the analyses by the laboratory. Materials were from 12 healthy volunteers of both genders, aged between 25 and 35 years, who were not taking any medication or toxic substance, chosen according to the research criteria. The Informed Consent Form (ICF) was prepared, and the experimental protocol was approved by the Ethics in Research Committee (ERC) (Process 14382719.4.0000.5401). This involved adding 2 mL of hyposaline solution (0.18%), 1 mL of sodium phosphate buffer (0.1 M, pH 7.4), 1 mL of analyzed samples of the fractions tested at three different concentrations (200, 400, and 600 µg mL-1), and 0.5 mL of HRBC solution. The hemoglobin content in the suspension was measured using a spectrophotometer at a wavelength of 560 nm. 1 mL of dexamethasone 40 µg mL-1 served as a positive control, while 1 mL of 0.9% saline solution was the negative control [19]. The anti-inflammatory activity was calculated using Equation 1.
Anti-inflammatory Activity (%) = (E0 - ET)/E0 × 100 (1)
Where:
E0 - Absorbance of the negative control group
ET - Absorbance of treatment groups
Next, the tests spreading and the phagocytosis were performed with murine macrophages of the 264.7 RAW (ATCC TIB-71). The macrophage strain was thawed and cultivated in a cell culture flask with Dulbecco’s Modified Eagle Medium (DMEM) and Ham’s F-12 culture medium at 37 °C, 5% CO2. The anti-inflammatory efficacy was performed when the culture reached about 70% - 80% confluence. At this moment cells were harvested using a cell scraper, counted in a Neubauer chamber, and centrifuged at 1500 rpm for 5 min. Then, the supernatant was discarded, and the cells were resuspended in a culture medium to reach the desired concentration for each experiment.
The spreading by macrophages was determined using the method described by Bastos, et al. (2012). Samples of B. trimera and E. arvense fractions were analyzed at three different concentrations (200, 400, and 600 µg mL-1) [19]. Dexamethasone 40 µg mL-1 was used as a positive control and 0.9% saline solution was used as a negative control. Slides were prepared and examined under an optical microscope at 400x magnification, with a total count of 100 cells. This test was conducted in triplicate [20]. The inhibition of spreading was calculated using the following expression (equation 2).
Spreading Inhibition (%) = (E0 - ET)/E0 × 100 (2)
Where:
E0 - The average number of spread cells in the negative control group
ET - The average number of spreading cells in the treated groups.
The phagocytosis assay performed by macrophages followed the methodology described by Azedo, et al. (2012). Samples of B. trimera and E. arvense fractions were analyzed at three different concentrations (200, 400 and 600 µg mL-1). As a positive control, dexamethasone was used (concentration of 40 µg mL-1), and saline solution (0.9%) was the negative control. The prepared slides were examined under an optical microscope at 400x magnification, with a total count of 100 cells. The experiment was conducted in triplicate, with the Phagocytosis Inhibition (PI) calculated according to the following expression (equation 3) [21].
Phagocytosis Inhibition, PI (%) = (E0 - ET)/E0 × 100 (3)
Where:
E0 - Average number of cells in the negative control group that phagocytosed Zymosan particles
ET - The average number of cells in the treated groups that phagocytosed Zymosan particles.
Statistical analysis
The data of the in vitro experiments were expressed in terms of mean ± standard deviation. Statistical analysis was performed using BioEstat® (version 5.0) software (Brazil). To verify the statistical differences between the groups a one-way analysis of variance (ANOVA) was performed according to the experimental protocol, followed by Tukey’s multiple comparison test. For all analyzes a p - value of < 0.05 was considered significant.
In the chemistry of natural products, the diverse potential of special metabolites is utilized to promote the bioprospecting of plant molecules or fractions with the goal of expanding the pharmacological applicability of herbal medicines [22,23]. The Brazilian flora is an invaluable source of compounds, constituting approximately 25% of the world’s total. Therefore, it is believed that the Brazilian flora holds great potential in providing compounds for various uses, especially in traditional medicine. However, some plants lack in-depth studies on their pharmacological applications. As a result, the use of extracts, infusions, fresh consumption, or essential oils from various plants, such as E. arvense and B. trimera, requires further detailed studies to scientifically demonstrate the biological role of certain compounds or mixtures of compounds [24].
Based on the vast variety of medicinal plants in Brazil, the extensive genus called Equisetum stands out, comprised of around 30 different species, all of which have uses in folk medicine. Furthermore, this genus can be considered one of the oldest in the world [24]. Among the plants of the Equisetum genus, Equisetum arvense L. stands out, a plant popularly known as “cavalinha”, which is generally used to combat fluid retention. According to a study by Boeing (2021), E. arvense L. showed results on its diuretic effect, treatment of genitourinary diseases, inflammation, wound healing, and other benefits [24]. Although its diuretic effect has been proven in animal models and clinical trials, more studies are needed to prove its effectiveness in humans in terms of anti-inflammatory activities. In another study conducted by Monte (2004), the antinociceptive and anti-inflammatory effects of E. arvense in extract form were evaluated in mice at dosages of 10, 25, 50, and 100 mg.kg-1. Studies indicate that the use of the extract contains antinociceptive effects and anti-inflammatory properties [25].
The species Baccharis trimera, also known as “carqueja”, is a medicinal plant widely found in South America and used in ethnopharmacology to treat diseases associated with liver and gastric problems [11]. B. trimera also exhibits cardioprotective effects, possibly due to its lipid-lowering action and inhibition of free radicals’ production [26]. Gene and collaborators (1996) describe a potential anti-inflammatory action in aqueous extracts of B. trimera, comparing it with the action of non-steroidal anti-inflammatory drugs [27]. Other biological activities include gastric protection, hepatoprotection, weight loss effects, and anthelmintic, antifungal, antiparasitic, and antiviral properties, among others [15]. In this sense, E. arvense and B. trimera are excellent sources of metabolites, and the need for research is evident, especially in exploring the anti-inflammatory activities of their fractions, which have been minimally studied in the literature.
The essential oils obtained from E. arvense (EA-EO) and B. trimera (BT-EO) are colorless and green, respectively, with a relative percentage of 1.7% and 0.5% (w/w). We also chose to obtain classes of compounds generally of high polarity and/or high molecular weight, such as alkaloids, triterpenes, organic acids, and flavonoids, among others. The extracts of E. arvense and B. trimera were both presented in the form of brown oils, with extraction yields of 2.7% and 6.2% (w/w). According to Oliveira and collaborators (2012), the essential oil of B. trimera was obtained at a percentage of 0.5% (w/w), which corroborates the mass obtained in our extraction. In another study by Oliveira and collaborators (2012), the aqueous extract of B. trimera was obtained with a yield of 14.1% (w/w), demonstrating the low effectiveness in obtaining compounds from the ethanolic extract of the plant specimen [28-30]. Comparing the percentages of volatile fractions obtained with the literature, reports reveal a percentage of 0.2% for E. arvense [31]; and for the extract, Monte (2004) and collaborators describe a percentage of 14.0% (w/w) yield [25]. These data indicate that the extractions were quite efficient for the organic phases obtained, however, it is necessary to study whether obtaining an ethanolic extract is appropriate, or even whether the plant itself can develop different amounts/natures of metabolites depending on its region, climate, treatment and other aspects [32].
The inflammation process involves the release of several hydrolytic enzymes from the lysosome, which can cause damage to surrounding tissues and organelles, generating a wide range of disorders [33]. One of the viable alternatives to access data regarding potential inflammatory processes is the assay stabilization of the red blood cell membrane. We chose to use four fractions obtained in different concentrations to understand the dose and biological response. These fractions were applied at concentrations of 200, 400, and 600 µL mL-1 for the volatile fractions obtained in the form of Essential Oil (EO) and non-volatiles obtained in the form of ethanolic extract (EE) of E. arvense (EA) and B. trimera (BT). Data analysis suggests moderate stabilization of the red blood cell membrane by the BT-EE fraction, which is the most active fraction evaluated. Initially, a slight correlation of increased stabilization of red blood cell membranes at higher concentrations is noticeable, as seen at a concentration of 600 µg mL-1 (Absorbance of 65.50 ± 0.13 %, p < 0.05). These data align with Oliveira and collaborators, who used Carrageenan-induced Pleurisy to identify the anti-inflammatory potential in B. trimera fractions [28-30].
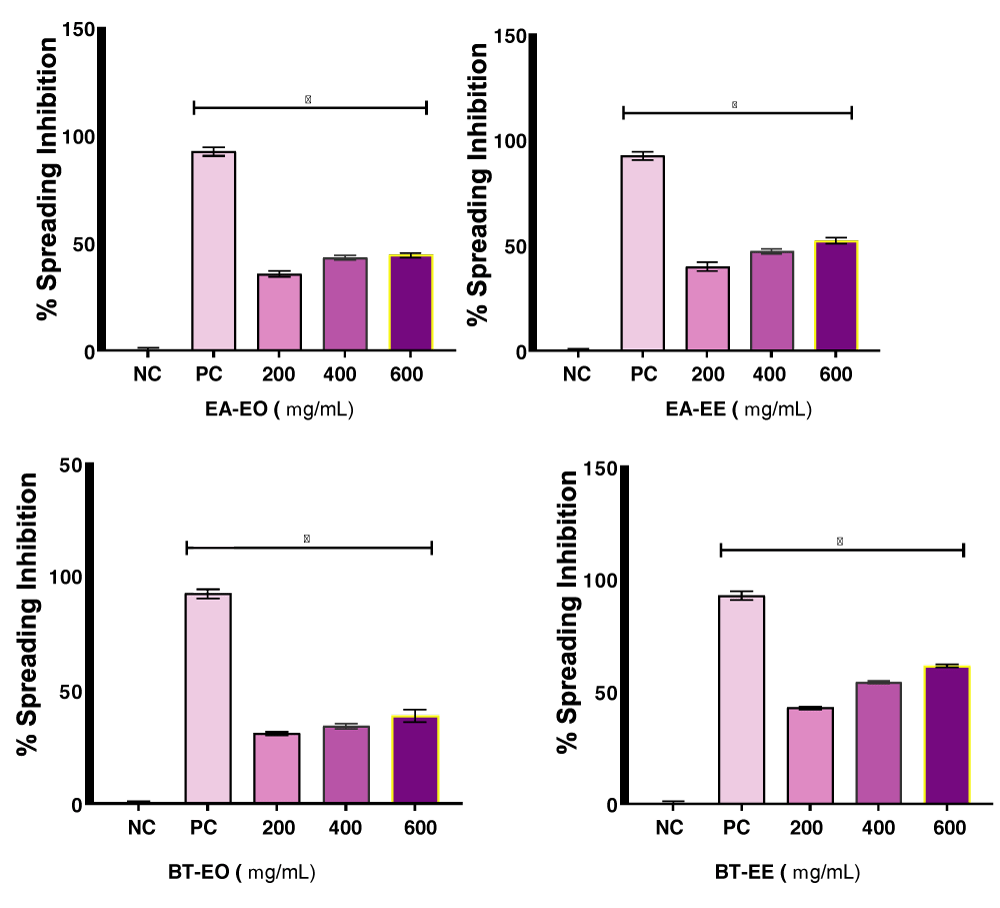
Figure 1 depicts spreading inhibition carried out by macrophages compared to the positive control (Dexamethasone 40 µg mL-1). The functional analysis of the spread of mononuclear phagocytes is crucial in the study of the inflammatory process. The functional capacity of these cells, which participate in processes such as lysis and engulfment of particles or microorganisms, generation of hydrogen peroxide, adhesion, and emission of microvilli, is closely influenced by inflammation [20,31]. Although they differ significantly from the positive control, the spread analysis does not show drastic differences between the fractions. It is evident that the only fraction showing a spread greater than 50% is BT-EE, supporting the red blood cell stabilization data (Table 1). Data from this experiment for E. arvense and B. trimera are being reported for the first time.
Figure 1: Evaluation of the spreading inhibition carried out by macrophages in EO and EE fractions of E. arvense and B. trimera. NC (Negative Control) = Physiological solution 0.9%; PC (Positive control) = Dexamethasone 40 µg mL-1
| Table 1: Evaluation of the stabilization of the red blood cell membrane in EO and EE fractions of E. arvense and B. trimera. | ||
| Treatments | % Absorbance | Standard Deviation |
| Negative Control* | 0 | 0 |
| Positive Control** | 98.97 | 0.34 |
| EA-EO - 200 µg mL-1 | 37.22 | 0.06 |
| EA-EO - 400 µg mL-1 | 40.53 | 0.42 |
| EA-EO - 600 µg mL-1 | 44.50 | 0.12 |
| EA-EE - 200 µg mL-1 | 39.23 | 0.05 |
| EA-EE - 400 µg mL-1 | 46.82 | 0.01 |
| EA-EE - 600 µg mL-1 | 58.60 | 0.23 |
| BT-EO - 200 µg mL-1 | 45.87 | 0.02 |
| BT-EO - 400 µg mL-1 | 47.80 | 0.02 |
| BT-EO - 600 µg mL-1 | 65.50 | 0.13 |
| BT-EE - 200 µg mL-1 | 40.11 | 0.01 |
| BT-EE - 400 µg mL-1 | 44.15 | 0.00 |
| BT-EE - 600 µg mL-1 | 47.52 | 0.03 |
| * Negative Control = Physiological solution 0.9%; ** Positive control = Dexamethasone 40 µg mL-1. | ||
The action of the phagocytic cell is an important factor in determining the immune processes. The ability of phagocytes to engulf Zymosan particles compared to a positive control is an indicator of anti-inflammatory capacity [20]. Table 2 demonstrates a relationship between the treatment of essential oils and ethanolic extract from B. trimera and E. arvense at concentrations of 200 to 600 µg mL-1 and the phagocytosis inhibition carried out by macrophages (PI) compared to a positive control (Dexamethasone 40 µg mL-1) and a negative control. The data corroborates the other two experiments and can be considered promising for BT-EE 600 µg mL1 (p < 0.05).
| Table 2 : Assessment of phagocytosis inhibition (PI) performed by macrophages on EO and EE fractions of E. arvense and B. trimera. | ||
| Treatments | % PI | Standard Deviation |
| Negative Control* | 0 | 0 |
| Positive Control** | 73.64* | 1.61 |
| EA-EO - 200 µg mL-1 | 31.16* | 0.56 |
| EA-EO - 400 µg mL-1 | 34.58* | 1.45 |
| EA-EO - 600 µg mL-1 | 46.37* | 1.12 |
| EA-EE - 200 µg mL-1 | 51.60* | 2.00 |
| EA-EE - 400 µg mL-1 | 54.57* | 1.93 |
| EA-EE - 600 µg mL-1 | 58.73* | 1.15 |
| BT-EO - 200 µg mL-1 | 25.04* | 3.59 |
| BT-EO - 400 µg mL-1 | 29.87* | 1.69 |
| BT-EO - 600 µg mL-1 | 38.12* | 1.87 |
| BT-EE - 200 µg mL-1 | 46.43* | 0.44 |
| BT-EE - 400 µg mL-1 | 67.60* | 2.52 |
| BT-EE - 600 µg mL-1 | 73.44* | 1.39 |
| * Negative Control = Physiological solution 0.9%; ** Positive control = Dexamethasone 40 µg mL-1. | ||
It was observed that all treatments with the tested fractions showed significant differences (p < 0.05) compared to the negative control. Upon comparing the experiments, BT-EE is a promising fraction when compared to the positive control, as outlined in Tables 1,2. This corroborates with the literature, specifically a study conducted by Gene and collaborators (1996) which describes the anti-inflammatory activity of B. trimera [27]. This activity is attributed, in principle, to the prevention of prostaglandin biosynthesis through the inhibition of cyclooxygenase, as well as the fraction’s ability to reduce dextran-induced swelling [33,34]. This study is a preliminary basis, but further access to information is needed regarding the toxicity of the fractions, their chemical identification, quantification of other inflammation modulators, mainly on inflammation-related genes in isolated cell populations, and the pharmacodynamic response of isolated cell populations with protein-based assays. Up to now, we infer the use of therapeutic plants, such as B. trimera, can influence immune reactions and lead to anti-inflammatory effects. The promising results of B. trimera make it a valuable therapeutic plant for further future studies.
In the present study, essential oils and ethanolic extracts of E. arvense and B. trimera were obtained. The stabilization of the red blood cell membrane, inhibition of spreading, and phagocytosis process by macrophages were evaluated. In all experiments, we observed that the most polar fraction of B. trimera (BT-EE) exhibited significant anti-inflammatory activity compared to positive controls. Additionally, a moderate anti-inflammatory activity in vitro was found for the essential oils of both species, as well as for the ethanolic extract of E. arvense. These results suggest the need for further study on the mode of action and biochemical properties of the investigated fractions to identify potential molecules that provide anti-inflammatory activity in the natural product. New studies are being conducted to evaluate the cytotoxicity of the promising fraction, and the antioxidant activity of BT-EE, and determine the chemical constitution through LC-MSn and fraction isolation processes are underway.
The authors would like to thank Pró-Reitoria de Pesquisa, Pós-Graduação e Inovação – PROPPI-IFGoiano (grant number 23216.000929.2022-22) IF Goiano and Unesp – Campus Assis for the financial support.
AFM and CFV worked in obtaining the natural products and acted in anti-inflammatory data analysis. JARF and LS acted in an anti-inflammatory analytical process. MVVR classified the botanical species. HJD worked on the conception, discussion of results, writing of the manuscript, revision, and approval of the final version of the manuscript. All authors agree with the submission of the manuscript and declare that they have not submitted it to another journal during the review process.
- Megha KB, Joseph X, Akhil V, Mohanan PV. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine. 2021; 91:153712.
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008 Jul 24;454(7203):428-35. doi: 10.1038/nature07201. PMID: 18650913.
- Arulselvan P, Fard MT, Tan WS, Gothai S, Fakurazi S, Norhaizan ME, Kumar SS. Role of Antioxidants and Natural Products in Inflammation. Oxid Med Cell Longev. 2016;2016:5276130. doi: 10.1155/2016/5276130. Epub 2016 Oct 10. PMID: 27803762; PMCID: PMC5075620.
- Azab A, Nassar A, Azab A. Anti-inflammatory activity of natural products. Molecules. 2016; 21(10):1-19.
- Sharif K, Watad A, Bragazzi NL, Lichtbroun M, Amital H, Shoenfeld Y. Physical activity and autoimmune diseases: Get moving and manage the disease. Autoimmun Rev. 2018 Jan;17(1):53-72. doi: 10.1016/j.autrev.2017.11.010. Epub 2017 Nov 3. PMID: 29108826.
- Zhao H, Guo Q, Li B, Shi M. The Efficacy and Safety of Ginkgo Terpene Lactone Preparations in the Treatment of Ischemic Stroke: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front Pharmacol. 2022 Mar 18;13:821937. doi: 10.3389/fphar.2022.821937. PMID: 35392576; PMCID: PMC8982077.
- Braquet P, Hosford D. Ethnopharmacology and the development of natural PAF antagonists as therapeutic agents. J Ethnopharmacol. 1991 Apr;32(1-3):135-9. doi: 10.1016/0378-8741(91)90111-p. PMID: 1881152.
- Castro Oliveira HW, Viveiro AA. Cerrado and medicinal plants: Some reflections on use and conservation. Ens. Amb Health. 2013; 5(3):102-120.
- Corrêa M, Melo G, Costa S. Natural products from plant origin potentially usefull in the asthma therapy. Natural products from plant origin potentially usefull in the asthma therapy. Rev Bras Farmacogn. 2008; 18(SUPPL):785-797.
- Kuklinski C. Pharmacognosy study of drugs and medicinal substances of natural origin. Barcelona: Omega. 2003.
- Newman DJ, Cragg GM. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J Nat Prod. 2020 Mar 27;83(3):770-803. doi: 10.1021/acs.jnatprod.9b01285. Epub 2020 Mar 12. PMID: 32162523.
- Carneiro D, Jardim T, Araújo Y, Arantes A, Souza A, Barroso W, Souza A, Cunha L, Cirilo H, Bara M, Jardim P. Equisetum arvense: New evidences supports medical use in daily clinic. Pharmacogn Rev. 2019; 13(26):50-58.
- Rabelo A, Costa D. A review of biological and pharmacological activities of Baccharis trimera. Chem. Biol. Interact. 2018; 296: 65-75.
- Alcoba AET, Melo DC, de Andrade PM, Dias HJ, Pagotti MC, Magalhães LG, Júnior WGF, Crotti AEM, Miranda MLD. Chemical composition and in vitro antileishmanial and cytotoxic activities of the essential oils of Ocotea dispersa (Nees) Mez and Ocotea odorifera (Vell) Rohwer (Lauraceae). Nat Prod Res. 2018 Dec;32(23):2865-2868. doi: 10.1080/14786419.2017.1385007. Epub 2017 Oct 12. PMID: 29022353.
- Vieira TM, Dias HJ, Medeiros TCT, Grundmann CO, Groppo M, Heleno VCG, Martins CHG, Cunha WR, Crotti AEM, Silva EO. Chemical composition and antimicrobial activity of the essential oil of Artemisia absinthium Asteraceae leaves. J. Essent. Oil-Bear. Plants. 2017; 20(1): 123-131.
- Dias HJ, Vieira TM, Carvalho CE, Aguiar GP, Wakabayashi KA, Turatti ICC, Willrich GB, Groppo M, Cunha WR, Martins CHG, Crotti AEM. Screening of selected plant-derived extracts for their antimicrobial activity against oral pathogens. Int J Complement Alt Med. 2027; 6(2017): 00188.
- Singh B, Brahma M, Gurung J. An Investigation of traditional uses and anti-inflammatory property of Clematis buchananiana De Candolle and Tupistra nutans Wall. Ex Lindl.: Native ethnomedicinal plants from Sikkim, India. Indian J. Tradit. Knowl. 2020; 19:719–727.
- Fracasso JAR, Ibe MB, da Costa LTS, Guarnier LP, Viel AM, Brito GR, Parron MC, Pereira AEDS, Pegorin Brasil GS, Farias Ximenes V, Fraceto LF, Malacrida Mayer CR, Ribeiro-Paes JT, Ferreira FY, Zoppe NA, Santos LD. Anti-Inflammatory Effect and Toxicological Profile of Pulp Residue from the Caryocar Brasiliense, a Sustainable Raw Material. Gels. 2023 Mar 16;9(3):234. doi: 10.3390/gels9030234. PMID: 36975683; PMCID: PMC10048353.
- Ananthi T, Chitra M. Screening of in vitro anti-inflammatory activity of Michelia champaca Linn. Flowers. Asian J. Pharm. Clin. Res. 2013; 6(5):15-16.
- Bastos C, Blagitz M, Souza F, Batista C, Stricagnolo C, Azedo M, Libera A. Cell viability, phagocytosis and spreading of mononuclear phagocytes, and release of hydrogen peroxide by leukocytes from healthy and infected bovine mammary glands. Research Vet. Bras. 2012; 32(9):850-854.
- Azedo M, Blagitz M, Souza F, Benesi F, Libera A. Functional evaluation of bovine monocytes naturally infected by bovine leukosis virus. Arq Bras Med Vet Zootec. 2011; 63(5):1131-1140.
- Khan R. Natural products chemistry: The emerging trends and prospective goals. Saudi Pharm J. 2018; 26(5):739-753.
- Dias HJ, Fernandes CP, Hussain H. Editorial: Analytical chemistry applied to natural products: trends and challenges. Front Pharmacol. 2023 Jul 4;14:1235224. doi: 10.3389/fphar.2023.1235224. PMID: 37469876; PMCID: PMC10352822.
- Boeing T, Tafarelo Moreno KG, Gasparotto Junior A, Mota da Silva L, de Souza P. Phytochemistry and Pharmacology of the Genus Equisetum (Equisetaceae): A Narrative Review of the Species with Therapeutic Potential for Kidney Diseases. Evid Based Complement Alternat Med. 2021 Mar 5;2021:6658434. doi: 10.1155/2021/6658434. PMID: 33747109; PMCID: PMC7954623.
- Do Monte FH, dos Santos JG Jr, Russi M, Lanziotti VM, Leal LK, Cunha GM. Antinociceptive and anti-inflammatory properties of the hydroalcoholic extract of stems from Equisetum arvense L. in mice. Pharmacol Res. 2004 Mar;49(3):239-43. doi: 10.1016/j.phrs.2003.10.002. PMID: 14726218.
- Souza MMQ, Silva GRD, Cola IM, Silva AO, Schaedler MI, Guarnier LP, Palozi RAC, Barboza LN, Menetrier JV, Froelich DL, Auth PA, Veiga AA, Souza LM, Lovato ECW, Ribeiro-Paes JT, Gasparotto Junior A, Lívero FADR. Baccharis trimera (Less.) DC: An Innovative Cardioprotective Herbal Medicine Against Multiple Risk Factors for Cardiovascular Disease. J Med Food. 2020 Jun;23(6):676-684. doi: 10.1089/jmf.2019.0165. Epub 2019 Nov 8. PMID: 31702422.
- Gené RM, Cartaña C, Adzet T, Marín E, Parella T, Cañigueral S. Anti-inflammatory and analgesic activity of Baccharis trimera: identification of its active constituents. Planta Med. 1996 Jun;62(3):232-5. doi: 10.1055/s-2006-957866. PMID: 8693035.
- Oliveira H. Cerrado and medicinal plants: some reflections on use and conservation. Ens. Amb Health. 2013; 5(3):102-120.
- de Oliveira CB, Comunello LN, Lunardelli A, Amaral RH, Pires MG, da Silva GL, Manfredini V, Vargas CR, Gnoatto SC, de Oliveira JR, Gosmann G. Phenolic enriched extract of Baccharis trimera presents anti-inflammatory and antioxidant activities. Molecules. 2012 Jan 23;17(1):1113-23. doi: 10.3390/molecules17011113. PMID: 22269829; PMCID: PMC6268486.
- de Oliveira RN, Rehder VL, Santos Oliveira AS, Júnior ÍM, de Carvalho JE, de Ruiz AL, Jeraldo Vde L, Linhares AX, Allegretti SM. Schistosoma mansoni: in vitro schistosomicidal activity of essential oil of Baccharis trimera (less) DC. Exp Parasitol. 2012 Oct;132(2):135-43. doi: 10.1016/j.exppara.2012.06.005. Epub 2012 Jul 4. PMID: 22771865.
- Gu H, Yi T, Lin P, Hu J. Study on essential oil, antioxidant activity, anti-human prostate cancer effects, and induction of apoptosis by Equisetum arvense. Open Chem. 2022; 20(1):1187-1195.
- Ahmed S, Griffin T, Kraner D, Schaffner M, Sharma D, Hazel M, Leitch A, Orians C, Han W, Stepp J, Robbat A, Matyas C, Long C, Xue D, Houser R, Cash S. Environmental factors variably impact tea secondary metabolites in the context of climate change. Front. Plant Sci. 2019; 10(939):939-956
- Yadati T, Houben T, Bitorina A, Shiri-Sverdlov R. The Ins and Outs of Cathepsins: Physiological Function and Role in Disease Management. Cells. 2020 Jul 13;9(7):1679. doi: 10.3390/cells9071679. PMID: 32668602; PMCID: PMC7407943.
- Paul EL, Lunardelli A, Caberlon E, de Oliveira CB, Santos RC, Biolchi V, Bastos CM, Moreira KB, Nunes FB, Gosmann G, de Oliveira JR. Anti-inflammatory and immunomodulatory effects of Baccharis trimera aqueous extract on induced pleurisy in rats and lymphoproliferation in vitro. Inflammation. 2009 Dec;32(6):419-25. doi: 10.1007/s10753-009-9151-1. PMID: 19756999.