More Information
Submitted: March 07, 2024 | Approved: April 05, 2024 | Published: April 08, 2024
How to cite this article: de Andrade RMZ, de Paixão Santos B, Silva RMF, Silva MG, de Sousa Oliveira I, et al. Antibacterial Screening of Lippia origanoides Essential Oil on Gram-negative Bacteria. Arch Pharm Pharma Sci. 2024; 8: 024-028.
DOI: 10.29328/journal.apps.1001053
Copyright License: © 2024 de Andrade RMZ, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and repro-duction in any medium, provided the original work is properly cited.
Keywords: Essential oil; Rosemary-pepper; Lippia organoids; Antibacterial activity
Antibacterial Screening of Lippia origanoides Essential Oil on Gram-negative Bacteria
Rodrigo Marcelino Zacarias de Andrade1, Bernardina de Paixão Santos2, Roberson Matteus Fernandes Silva1, Mateus Gonçalves Silva3*, Igor de Sousa Oliveira2, Sávio Benvindo Ferreira4 and Rafaelle Cavalcante Lira4
1Nursing Student, Academic Unit of Nursing (UAENF), Teacher Training Center, Federal University of Campina Grande (UFCG-0), Brazi
2Medical Student, Academic Unit of Life Sciences (UACV), Teacher Training Center, Federal University of Campina Grande (UFCG-0), Brazil
3Bachelor’s degree in Agroecology from the Federal Institute of Paraíba, Master’s degree in Agroindustrial Systems from the Federal University of Campina Grande, Bachelor’s student in Biological Sciences at the Federal University of Paraíba, Brazil
4Doctor Professor, Academic Unit of Life Sciences (UACV), Teacher Training Center (CFP), Federal University of Campina Grande (UFCG-0), Brazil
*Address for Correspondence: Mateus Gonçalves Silva, Bachelor’s degree in Agroecology from the Federal Institute of Paraíba, Master’s degree in Agroindustrial Systems from the Federal University of Campina Grande, Bachelor’s student in Biological Sciences at the Federal University of Paraíba, Brazil, Email: [email protected]
Essential oils (EO) are extracted from different plant species and can be present in different plant organs. Rosemary-pepper EO is composed of around 50% to 70% thymol, a phenolic compound proven to be active against fungi and bacteria. The active components present in these compounds can affect the vital functionality of bacterial cells, leading to protein denaturation and cell lysis. Therefore, the present study aims to evaluate in vitro the antibacterial potential of Lippia origanoides EO against gram-negative bacteria. This is an exploratory study, with a technical-experimental procedure, with a quantitative approach, carried out at the Federal University of Campina Grande. The strains used were Pseudomonas aeruginosa ATCC 27853, Proteus mirabilis ATCC 25933, and Escherichia coli ATCC 25922, using concentrations of 1024, 512, 256, and 128 μg/ml using the disc diffusion method in triplicate. After the incubation period, the formation of halos of bacterial growth inhibition was not observed. There are possible causes for the lack of antibacterial activity of the EO concerning the strains of gram-negative bacteria used in the study, including the possibility of not containing components with antibacterial properties in concentrations sufficient for the expected activity at the concentrations tested. Based on the results obtained, the Rosemary-Pepper EO (Lippia organoids) did not demonstrate antimicrobial activity against the gram-negative bacteria used in the study. Therefore, the development of new research with Lippia origanoides essential oil with gram-positive bacteria is suggested.
Essential Oils (EO) are extracted from more than 17,500 plant species and are present in different plant organs such as flowers, leaves, stems, bark, seeds, and fruits [1]. Economically, essential oils have been highlighted in terms of their abundance in the environment and the demand for natural products has increased considerably in recent years, therefore becoming known and valued products [2]. Furthermore, studies show that essential oils are substances with high power to combat microorganisms and are low cost, easily accessible, and less toxic in combating illnesses [3,4].
Pepper rosemary is a plant that belongs to the Verbenaceae family, which contains several medicinal species of economic interest, and has been used in traditional Brazilian medicine due to its diverse biological activities [5,6]. This species is predominantly found in the North and Northeast regions of Brazil, mainly in the Amazon region, where it is used to treat skin lesions and inflammation of the upper airways, among other therapeutic purposes [7-9].
The eventual emergence of antimicrobial resistance to conventional antibiotics available on the market has become a serious public health problem, directly harming healthcare, and thus raises the need for studies of alternative substances capable of obtaining antimicrobial activity [9-12]. In this way, research on the antimicrobial potential of essential oils gains space and relevance for combating these diseases [13,14].
Rosemary-pepper EO contains around 50% to 70% thymol, a phenolic compound with proven activity against fungi and bacteria. Pepper rosemary is considered a plant with great potential for the development of new products in the pharmaceutical and cosmetic sectors, due to its medicinal properties. Such potential is particularly relevant in a scenario of dissemination of antibiotic-resistant bacterial strains, which has been a challenge for public health. In this context, rosemary pepper emerges as a promising option for the development of new compounds with antimicrobial activity, which may be useful in combating infections caused by these resistant strains [15-17].
In laboratory studies in Colombia, the antibacterial activity of L. origanoides was shown to be effective at concentrations of > 900 μg/ml for thymol, in bacteria such as E. coli, K. pneumoniae, Acinetobacter spp., and S. aureus [18]. According to studies, the active ingredients in essential oils can denature proteins, causing the bacteria to die, but the effect of essential oils depends on some variants, such as the place and time in which it was collected, influencing the concentration of antimicrobial compounds that will act on bacteria [19-22].
Therefore, this study aims to evaluate the bactericidal potential of Lippia origanoides essential oil in vitro in contact with gram-negative bacteria.
Type of study and research location
The research is an exploratory study, using a technical-experimental procedure, with a quantitative approach, to evaluate the antibacterial potential of Lippia origanoides essential oil in vitro in contact with gram-negative bacteria. The laboratory tests were carried out at the CT-INFRA Microbiology Laboratory at the Teacher Training Center of the Federal University of Campina Grande, Cajazeiras campus, in the state of Paraíba.
Test substance
Essential oil taken from the leaves of the rosemary-pepper plant (Lippia origanoides) was used. This was produced at Fazenda Lagoa Vermelha, a location belonging to NatEssential, the company responsible for manufacturing the oil. The oil was obtained through purchase on the website https://www.natessential.com.br/alecrim-pimenta.html. In its chemical composition, according to the information present in the product’s technical sheet, two components are mainly found: Thymol (50.40g/100g) and Carvacrol (0.3g/100g) [23].
A solution of this compound was made by diluting this oil in 2% Tween 80% and 10% DMSO, as well as using sterile distilled water to obtain the desired concentrations.
Culture mediums
To carry out the tests, the culture medium used was Mueller-Hinton Agar and BHI Broth. Before being manipulated, these media were solubilized in distilled water and sterilized in an autoclave at 121°C for 15 minutes.
Microorganisms
Gram-negative bacterial strains from the ATCC (American Type Culture Collection) were used, with the species being Pseudomonas aeruginosa ATCC 27853; Proteus mirabilis ATCC 25933, and Escherichia coli ATCC 25922.
Bacterial inoculums
The bacterial strains were inoculated into BHI broth and kept in an oven for 24 hours at 37°C. After the incubation period, minimal amounts of the suspension were applied to the sterile BHI solution in another tube, until it became cloudy. This suspension had a turbidity similar to the 0.5 McFarland scale, where it was adjusted with the help of the spectrophotometer.
Disk-diffusion method
Screening investigations of natural products for pharmacological activity are essential for the discovery of new medicines. The screening was carried out using the disk diffusion method, accepted by the FDA (Food and Drug Administration) and established as a standard by CLSI (Clinical and Laboratory Standards Institute) [24]. For this, an aliquot of 20 µL of the solution from the essential oil was used on 6 mm diameter filter paper discs, in the different concentrations chosen for the test, reducing from 1024 to 128 µg/mL. To recognize whether the solvents DMSO and Tween 80 caused any interference in the test results, a negative control was prepared where 20 µL of the solvents DMSO (10%) and Tween 80 (2%) were placed on paper discs and, to For the Fontrol, the antibacterial gentamicin (30 µcg) was used.
The plates were aseptically closed and incubated at 37 °C for 24 hours in a bacteriological oven so that readings could be carried out [24]. Finally, the presence or absence of halos was observed, where these were measured with the aid of a millimeter ruler.
Statistical analysis
All experiments were performed in triplicate. The results were subjected to statistical treatment using GraphPad
Prism® 5.0 software (GraphPad Software, Inc., San Diego, CA). The data obtained were subjected to analysis of variance (ANOVA) and expressed as the mean + standard deviation. Differences were evaluated using the paired t-test and were considered significant when p < 0.05.
Initially, the disk-diffusion method was applied, which is postulated by Bauer, characterized by diffusing an antimicrobial through the culture medium. After this, the plates of the diameters of the growth inhibition halos were read, as shown in Table 1.
| Table 1: Diameter of bacterial growth inhibition zones for O.E. Lippia origanoides, gentamicin, and control against strains of Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, Proteus mirabilis ATCC 25933 and strains of Pseudomonas aeruginosa ATCC 27853. | ||||||
| Microorganisms | Growth Inhibition Halo Diameter (mm) | |||||
| Lippia origanoides (µg/mL) | Gentamicin (µg) | *C | ||||
| 1024 | 512 | 256 | 128 | 10 | ||
| Pseudomonas aeruginosa ATCC 27853 |
U# | U# | U# | U# | 23,6 + 2,3 | U# |
| Escherichia coli ATCC 25922 |
U# | U# | U# | U# | 22 + 1 | U# |
| Proteus mirabilis ATCC 25933 |
U# | U# | U# | U# | 22 + 1 | U# |
| *C - Solvent/diluent control: Discs impregnated with a solution of DMSO (10%) and Tween 80 (2%); U# - It was not possible to visualize the formation of a halo of inhibition of bacterial growth in the concentration of the substance used in the disk diffusion method. |
||||||
Gentamicin is a natural antibiotic from the aminoglycoside class. It is a drug of first choice due to its low commercial cost, good stability, and low rate of pathogenic resistance. In this sense, its mechanism of action consists of binding to the cellular components of bacteria and inducing the production of abnormal proteins, which are acutely toxic to them. Furthermore, it has bactericidal activity against the following resistant microorganisms: Salmonella sp, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and gram-positive bacteria such as Streptococcus sp. and Staphylococcus sp [25-27].
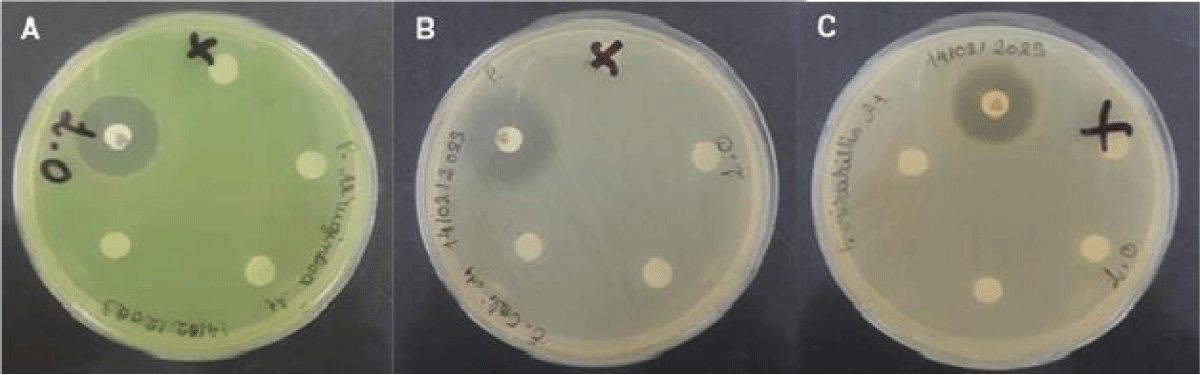
With this, it was possible to demonstrate that, given the concentrations tested, the rosemary-pepper essential oil did not present an antibacterial effect against the bacteria Pseudomonas aeruginosa ATCC 27853; Proteus mirabilis ATCC 25933 and Escherichia coli ATCC 25922 (Figure 1).
Figure 1: Result of the disc diffusion test performed with the essential oil Lippia origanoides in a plate with the bacterium Pseudomonas aeruginosa ATCC 27853 (A), Escherichia coli ATCC 25922 (B) and Proteus mirabilis ATCC 25933 (C).
The antibiotic gentamicin was used as a positive control, where sensitivity was observed for the bacteria selected in the study, in addition to the negative control, which showed that Tween 80 and DMSO do not have antimicrobial properties, as already predicted.
The main elements of essential oils have been widely studied for their effectiveness in treating infectious diseases. These substances are often compounds with antimicrobial action against a wide range of microorganisms, including those that are resistant to conventional antibiotics and antifungals. This range of use of essential oils is extensive and covers varied purposes [28].
Regarding the pharmacological action of these compounds, it is observed that they have antiseptic, carminative, stomachic, and expectorant properties, among others [29]. This antimicrobial activity is commonly related to compounds such as eugenol, allicin, thymol, and carvacrol. Such active ingredients, due to their hydrophobic characteristic, operate by cleaving the microbial cell wall, consequently losing their function [30].
The essential oil originating from Lippia origanoides mainly contains carvacrol and thymol, the latter being in high concentrations. It is reiterated that the thymol component has antimicrobial and bactericidal characteristics [31]. Santos Filho and collaborators observed in their studies that the composition of the plant species Lippia origanoides has around 23 compounds, with abundant components of thymol (47.2%), p-cymene (16.0%), and E-caryophyllene (11.3%)[32]. However, variations in the concentration of these compounds found in O.E in the Lippia species analyzed can also be found in the literature [33-36]. The effect of strongly lipophilic action, which is concentrated on the plasma membrane of the cell of this microorganism, harms cellular integrity, increasing its permeability. This results in the release of cytoplasmic contents, lysis, and death of cells [37].
In this sense, the active components from essential oils can penetrate the lipid structure of the bacteria’s cell wall, causing protein denaturation to occur, as well as the disruption of the cell membrane. Therefore, after cell lysis, the bacteria begin a process of death. Furthermore, the antibacterial activity of L. origanoides essential oils can alter protein synthesis, enzyme secretion, and the conversion of energy and nutrition, therefore altering the vital mechanisms for the functioning of microorganisms. Therefore, the antibacterial activity of rosemary-pepper essential oil lies in its ability to penetrate the cell walls of bacteria [22].
In the present study, antibacterial activity tests were carried out using Rosemary-pepper essential oil and its dilutions. The results obtained demonstrated that the essential oil tested did not exhibit antimicrobial activity against the bacteria studied. This lack of activity may be related to the insufficient concentration of the active components of the essential oil, as well as the specific characteristics of the bacterial strains used in the study, which may present resistance mechanisms that render the essential oil ineffective.
Although the essential oil in question did not show antibacterial activity against the gram-negative bacteria used in this study, this does not prevent further research to evaluate the potential antibacterial effect of this oil concerning gram-positive bacteria. It is important to highlight that the structural differences in the cell wall between these two types of bacteria can influence sensitivity to antimicrobial compounds, and, therefore, Rosemary-Pepper essential oil may present antibacterial activity against gram-positive bacteria.
It is proven that the action of essential oils may have a greater power of action on gram-positive bacteria, due to the greater interaction with the hydrophobic components of the cell wall concerning the gram-negative bacteria, which contain hydrophilic components. However, there is still a need to clarify some mechanisms, due to the divergence of results observed in studies available in the literature [38,39].
In this sense, a study carried out in Montes Claros, observed that rosemary-pepper oil caused bacteriostatic and bactericidal effects on species of Staphylococcus spp. and Streptococcus spp. isolated from the milk of sheep with mastitis. Furthermore, it is noteworthy that this action was observed at the highest concentration that was tested (150 µL/mL). There was also the formation of an inhibition halo in all bacteria used in the test, at a concentration of 150 µL/mL, through the disk diffusion test [36].
However, research carried out by Silva and collaborators demonstrates the effectiveness of Lippia origanoides essential oil for an antimicrobial effect using the Escherichia coli ATCC 25922 strain isolated from the environment, poultry inputs, cellulite lesions, and chicken liver. The divergences in results may be related to the concentration of the oil used in the studies, where in this study the highest concentration used was 1024 µg/mL, whereas in Silva’s research, the MIC found was 0.54 mg/ml, concluding that the effects of this oil can be found if there is an increase in concentrations, and consequently in the biological constituents of antimicrobial action [40].
That said, the divergence in antimicrobial activity observed between research available in the literature and the results of the present study may be due to the choice of bacterial species studied, where it is observed that the inhibition halos, and consequently the antibacterial effect, could be verified with gram-positive bacteria, contrary to what was observed in the gram-negative bacteria analyzed in this article, which exposes the greater bactericidal effect of Lippia origanoides oil on gram-positive bacteria [41].
Based on the results obtained, it was verified that the essential oil of Rosemary-Pepper (Lippia origanoides) did not present antimicrobial activity against the gram-negative bacteria Escherichia coli ATCC 25922, Proteus mirabilis ATCC 25933 and Pseudomonas aeruginosa ATCC 27853 at the tested concentrations of 1024, 512, 256 and 128 µg/mL. The lack of antibacterial activity suggests that the essential oil extracted from Lippia origanoides contains low concentrations of chemical substances with antibacterial effects against the strains examined. This suggests that the amount used in the experiment was not sufficient to demonstrate the antimicrobial effect of the methodology used.
- Nascimento AS, Tamiasso RSS, Turrini RNT, Calache ALSC, Poveda VB. Essential oils for healing and/or preventing surgical wound infection: systematic review USP Nursing School Magazine. 2022; 56: e20210442.
- Ribeiro MCMC, Oliveira VC, Virgens AP, Pereira T. Development of a natural cosmetic with lemongrass and pink pepper essential oils extracted using an enzymatic process. Research, Society and Development. 2022. 11(15): e308111537174
- Oliveira AFM, Silva FL, Morais FM. Atividade antimicrobiana de óleos essenciais frente a bactérias patogênicas de importância clínica. Research, Society and Development. 2022; 11(13): e448111335639
- OMS. Organização Mundial da Saúde. Traditional medicine: definitions. 2017.
- Oliveira DR, Leitão GG, Fernandes PD, Leitão SG. Ethnopharmacological studies of Lippia origanoides. Revista Brasileira de Farmacognosia. 2014; 24(2): 206-214.
- Teles S, Pereira JA, Oliveira LM, Malheiro R, Machado SS, Lucchese AM, Silva F. Organic and mineral fertilization influence on biomass and essential oil production, composition and antioxidant activity of Lippia origanoides H.B.K. Industrial Crops and Products. 2014; 59: 169-176.
- O’Leary N, Thode V. The Genus Glandularia (Verbenaceae) in Brazil. Annals of the Missouri Botanical Garden. 2016; 101(4): 699-749.
- Mpiana PT. Traditional uses, physical properties, phytochemistry and bioactivity of Lippia multiflora moldenke (Verbenaceae): a mini-review. Discovery Phytomedicine. 2020; 7(1): 19-26.
- Dadgostar P. Antimicrobial Resistance: Implications and Costs. Infect Drug Resist. 2019 Dec 20;12:3903-3910. doi: 10.2147/IDR.S234610. PMID: 31908502; PMCID: PMC6929930.
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022 Feb 12;399(10325):629-655. doi: 10.1016/S0140-6736(21)02724-0. Epub 2022 Jan 19. Erratum in: Lancet. 2022 Oct 1;400(10358):1102. PMID: 35065702; PMCID: PMC8841637.
- Rocha C, Reynolds ND, Simons MP. Resistencia emergente a los antibióticos: una amenaza global y un problema crítico en el cuidado de la salud [Emerging antibiotic resistance: a global threat and critical healthcare problem]. Rev Peru Med Exp Salud Publica. 2015 Jan-Mar;32(1):139-45. Spanish. PMID: 26102117.
- Prabu DL, Chandrasekar S, Ambashankar K, Dayal JS, Ebeneezar S, Ramachandran K, Vijayagopal P. Effect of dietary Syzygium cumini leaf powder on growth and non-specific immunity of Litopenaeus vannamei (Boone 1931) and defense against virulent strain of Vibrio parahaemolyticus. Aquac. 2018; 489: 9-20.
- de Melo ARB, Maciel Higino TM, da Rocha Oliveira ADP, Fontes A, da Silva DCN, de Castro MCAB, Dantas Lopes JA, de Figueiredo RCBQ. Lippia sidoides and Lippia origanoides essential oils affect the viability, motility and ultrastructure of Trypanosoma cruzi. Micron. 2020 Feb;129:102781. doi: 10.1016/j.micron.2019.102781. Epub 2019 Nov 12. PMID: 31830667.
- Herculano ED, Paula HCB, Figueiredo EAT, Dias FGB. Physicochemical and antimicrobial properties of nanoencapsulated Eucalyptus staigeriana essential oil. Lebensmittel-Wissenschaft undTechnologie-Food Science and Technology. 2015; 61(2): 484-491.
- Borugă O, Jianu C, Mişcă C, Goleţ I, Gruia AT, Horhat FG. Thymus vulgaris essential oil: chemical composition and antimicrobial activity. J Med Life. 2014;7 Spec No. 3(Spec Iss 3):56-60. PMID: 25870697; PMCID: PMC4391421.
- Baldim I, Tonani L, Kress MRZ, Oliveira WP. Lippia sidoides essential oil encapsulated in lipid nanosystem as an anti-Candida agent. Industrial Crops and Products. 2019; 127:73-81.
- Santos Filho LGAD, Reis RBD, Souza ASQ, Canuto KM, Brito ES, Castro KNC, Pereira AML, Diniz FM. Chemical composition and biological activities of the essential oils from Lippia alba and Lippia origanoides. An Acad Bras Cienc. 2023 Feb 10;95(1):e20220359. doi: 10.1590/0001-3765202320220359. PMID: 36790271.
- Quiguanás-Guarín ES, Agudeo JPB, López-Carvajal JE, Tabares YAR, Sanabria LP, Castaño-Osorio JC. In vitro cytotoxic activity of Lippia origanoides essential oil and its antibacterial effect against five bacteria of clinical importance. Natural Products Magazine. 2022; 5(2): 92-93.
- Dhouioui M, Boulila A, Chaabane H, Zina MS, Casabianca H. Seasonal changes in essential oil composition of Aristolochia longa L. ssp. paucinervis Batt. (Aristolochiaceae) roots and its antimicrobial activity. Industrial Crops and Products. 2016; 83: 301-306.
- Gasparetto A, Cruz AB, Wagner TM, Bonomini TJ, Correa R, Malheiros A. Seasonal variation in the chemical composition, antimicrobial and mutagenic potential of essential oils from Piper cernuum. Industrial Crops and Products. 2017; 95: 256-263.
- Ribeiro FP, Santana de Oliveira M, de Oliveira Feitosa A, Santana Barbosa Marinho P, Moacir do Rosario Marinho A, de Aguiar Andrade EH, Favacho Ribeiro A. Chemical Composition and Antibacterial Activity of the Lippia origanoides Kunth Essential Oil from the Carajás National Forest, Brazil. Evid Based Complement Alternat Med. 2021 Oct 19;2021:9930336. doi: 10.1155/2021/9930336. PMID: 34712353; PMCID: PMC8548111.
- Andrade MF, Silva IDL, Caetano VF, Vinhas GM, Almeida YMB. Advances in Food Science and Technology. Editora Científica Digital; Rosemary-pepper, orange and clove essential oils as natural antimicrobial additives: A narrative review. 2022; 19: 295-308.
- Nat Essential. Technical sheet of Rosemary-Pepper essential oil.
- Bayot ML, Bragg BN. Antimicrobial Susceptibility Testing. 2022 Oct 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 30969536.
- Liu JW, Xu WQ, Zhu XY, Dai XQ, Chen SC, Han Y, Liu J, Chen XS, Yin YP. Gentamicin susceptibility of Neisseria gonorrhoeae isolates from 7 provinces in China. Infect Drug Resist. 2019 Aug 9;12:2471-2476. doi: 10.2147/IDR.S214059. PMID: 31496761; PMCID: PMC6691950.
- Liu Y, Chang H, Li Z, Feng Y, Cheng D, Xue J. Biodegradation of gentamicin by bacterial consortia AMQD4 in synthetic medium and raw gentamicin sewage. Sci Rep. 2017 Sep 8;7(1):11004. doi: 10.1038/s41598-017-11529-x. PMID: 28887556; PMCID: PMC5591267.
- Garcia R. Gentamicin in neonates: early and late effects on hearing (dissertation). Ribeirão Preto: University of São Paulo, Faculty of Medicine of Ribeirão Preto. 2023;
- Monteiro NF, Lima HMR, Silva FL, Sousa FCH, Silva WC, Reis LCM. Activity of Eucalyptus Globulus essential oil in controlling bacteria in the oral cavity. Research, Society and Development. 2021; 10(14): e59101420091.
- Rocha RRR, Ferreira WM, Gonçalves KAM. Benefits provided by the use of essential oils on the central nervous system and their antimicrobial activity: a literary review. Brazilian Journal of Development. 2022; 8(1):229-236.
- Reis JB, Figueiredo LA, Castorani GM, Veiga SMOM. Assessment of the antimicrobial activity of essential oils against food pathogens. Brazilian Journal of Health Review. 2020; 3(1): 342-363.
- Lima DS, Lima JC, Cavalcanti RMCB, Santos BHC, Lima IO. Study of the antibacterial activity of the monoterpenes thymol and carvacrol against strains of Escherichia coli that produce broad-spectrum β-lactamases. Pan-Amaz Health Magazine. 2017; 8(1): 17-21.
- Dos Santos Filho LGA; Dos Reis RB; Sousa ASQ; Canuto KM; De Brito ES. Chemical composition and biological activities of the essential oils from Lippia alba and Lippia origanoides. Annals of the Brazilian Academy of Sciences. 2023; 95(1): 1-13.
- Batista ES, Brandão FR, Majolo C, Inoue LAKA, Maciel PO, Oliveira MR, Chaves FCM, Chagas EC. Lippia alba essential oil as anesthetic for tambaqui. Aquaculture. 2018; 495: 545-549.
- Ebadi MT, Azizi M, Sefidkon F, Ahmadi N. Influence of different drying methods on drying period, essential oil content and composition of Lippia citriodora Kunth. Journal of Applied Research on Medicinal and Aromatic Plants. 2015 2(4): 182-187.
- Majolo C, Rocha SIB, Chaves FCM, Bizzo HR. Chemical composition of Lippia spp. essential oil and antimicrobial activity against Aeromonas hydrophila. Aquaculture Research. 2016; 48(5): 2380-2387.
- Machado TF, Nogueira NA, de Cássia Alves Pereira R, de Sousa CT, Batista VC. The antimicrobial efficacy of Lippia alba essential oil and its interaction with food ingredients. Braz J Microbiol. 2014 Aug 29;45(2):699-705. doi: 10.1590/s1517-83822014000200045. PMID: 25242961; PMCID: PMC4166302.
- Morão RP, Almeida AC, Martins ER, Prates JPB, Oliveira FD. Chemical constituents and pharmacological principles of rosemary-pepper essential oil (Lippia origanoides). Unimontes Scientific Magazine. 2020; 18(1): 74-81.
- Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Industrial Crops and Products 2014; 62: 250-264.
- Calo JR, Crandall PG, O' Bryan CA, Ricke SC. Essential oils as antimicrobials in food systems – A review. Food Control. 2015; 54: 111-119.
- Souza CN, Almeida AC, Xavier MTR, Costa JPR, Silva LMV, Martins ER. Antimicrobial activity of medicinal plants from the Minas Gerais cerrado against bacteria isolated from sheep with mastitis. Unimontes Scientific Magazine 2020; 19(2): 51-61.
- Silva LSGR, Juiz PJL, da Silva RM, Neiva GS, Teles S, da Silva IMM. Antimicrobial activity of Lippia origanoides essential oil against pathogenic Escherichia coli strains isolated from the chicken meat production process. Brazilian Journal of Medicinal Plants. 2018; 20: 395-402.