More Information
Submitted: October 19, 2023 | Approved: April 24, 2024 | Published: April 25, 2024
How to cite this article: Okiemute Rosa JA, Dummene Godwin N. Toxicity and Phytochemical Analysis of Five Medicinal Plants. Arch Pharm Pharma Sci. 2024; 8: 029-040.
DOI: 10.29328/journal.apps.1001054
Copyright License: © 2024 Okiemute Rosa JA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and repro-duction in any medium, provided the original work is properly cited.
Keywords: Genotoxicity; Cytotoxicity; BSLT
Toxicity and Phytochemical Analysis of Five Medicinal Plants
Johnson-Ajinwo Okiemute Rosa1* and Nyodee, Dummene Godwin2
1Department of Pharmaceutical/Medicinal Chemistry, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Nigeria
2Health Services Department, Ignatius Ajuru University of Education, Rumuolumeni, Port Harcourt, Nigeria
*Address for Correspondence: Johnson-Ajinwo Okiemute Rosa, Department of Pharmaceutical/Medicinal Chemistry, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Nigeria, Email: [email protected]
Recent studies have shown that long-term uses of herbs have been associated with a rise in morbidity and mortality rates. While most researches are focused on bioactivity investigations, the toxicity of many plants has not been reported. There is a paucity of data on the potential toxicity of the following plants: Harungana madagascariensis (HM), Pterocarpus osun (PO), Phoenix dactylifera (PD), Annona muricata (AM), and Rutidea parviflora (RP). To evaluate the toxicity of the above-mentioned plants; two tests were employed namely: The Brine shrimp lethality test (BSLT) and the Allium cepa test. A correlation between the oral acute toxicity assay in mice and the LC50 obtained from BSLT has been established. Allium cepa test measures the potential genotoxic effects of plant extracts exerted on the root meristem of A. cepa (onions). Plant extracts were administered in concentrations ranging from 100 to 2500 µg/ml to the A. cepa for 72 h to obtain their Mitotic Indices (MI) and EC50. Results of the MI at 2500 µg/ml for HM, PO, PD, AM, and RP were 3.75, 4.96, 5.96, 6.10, and 6.71 while 281.81, 398.11, 501.19, 630.96, and 707.9 µg/ml were obtained as the respective EC50 values. Furthermore, 10-1000 mcg/ml concentrations were administered in the BSLT and the obtained LC50 values were 116.3, 250, 581.5, 581.5, and 750 µg/ml. The toxicity result demonstrated that the five plants were moderately toxic, with RP exhibiting minimal toxicity values and thus potentially having a good safety profile. The phytochemical screening of these plants revealed the presence of some pharmacologically important classes of compounds that are abundant. Several bioactive and toxic compounds were identified in the GC-MS analysis for some of the plants.
Humans have relied heavily on plants and animals for food and their health needs. Plants produce over 10,000 different compounds which serve primarily to protect themselves from attacks by parasites, pathogens, and predators. A good number of the compounds could be potentially of use in the pharmaceutical industry [1].
Medicinal plants play an essential part in human cultural development globally. Medicinal plants have always been at the forefront of practically all cultures of civilization as they are considered a rich source of traditional medicines and modern medicines are also derived from them. These plants owe their activity to different phytochemicals namely: alkaloids, flavonoids, saponins, total phenols, tannins, glycosides, triterpenoids/steroids [1-3].
Traditional Herbal Medicines (THM) are naturally occurring plant-derived substances, with minute or no industrial treatment, and are useful for treating various illnesses in specific locations in the body or with healing ability to the whole human body [4]. With a greater percentage of the human population relying on herbs, plants play a dominant function in the health system, and this is particularly true in developing countries especially in Africa with an ancient history of herbal medicine use [5].
Toxic ingredients in herbs that are directly connected to the active chemicals’ contents in herbs, such as ephedrine-like alkaloids in Ma Huang, are thought to have internal effects. Toxins associated with external factors, including contamination, adulteration, and misidentification of herbal products, are linked with the foreign toxins content rather than the herbs themselves [6]. With the increase in the usage of herbs worldwide, either as a primary treatment or as a complementary and alternative medicine, the safety and efficacy of herbal remedies has become a health concern. To date, it is difficult to make reliable estimates of toxic effects due to herbal products, especially since (i) herbal preparations are perceived to be safe and therefore individuals using plant remedies may not know the products are responsible for some of the negative symptoms they have experienced. (ii) the communication gap between herbal medicinal users and their physicians on the use of those remedies; (iii) the presence of low-quality herbal medicines available, and (iv) the availability of counterfeit products [7,8].
Five medicinal plants used in this work are Annona muricata L. (leaves), Harungana madagascariensis Lam. (stem-bark), Phoenix dactylifera L. (seeds), Pterocarpus osun (heartwood) and Rutidea parviflora DC. (rootbark). These selected medicinal plants have a plethora of information on their bioactivities and however, a paucity of information on their toxicities.
Annona muricata
Annona muricata L. is a deciduous tropical evergreen standing tree with a height of about 16-26 feet, having an open, rounded canopy with large, lustrous, dark green leaves and it belongs to the Annonaceae botanical family. Some plant species in the Annonaceae family include; Annona muricata, Annona squamosa, Annona montana, Annona crassilfora, and Annona reticulate [9]. A. muricata is native to the warm tropics of South and Northern America and is now distributed in the tropical and subtropical regions worldwide and Africa, including Ghana, Cameroun, and Nigeria. A. muricata is popularly known as soursop, guanabana, graviola, sirsak and Brazilian pawpaw [9-12]. The fruit is unique with an inedible skin covered with many soft thorns of about 0.3 cm long and around 0.2 cm in diameter. Mature fruit is dark green, hard inside and out, and the flesh is white. When the fruit is ripe, the skin becomes soft and yellowish-green, and the flesh becomes creamy, juicy, and tender. It tastes like a combination of strawberries and pineapple, with a sour taste as opposed to the basic creamy taste reminiscent of banana and coconut [9,12,13].
Literature has reported various phytochemicals present in the leaves and they include alkaloids, flavonoids, terpenoids, phenols, saponins, phlbatannins, carbohydrates, steroids, and cardiac glycosides [4,8,14].
Ethno medicinal uses
The bark, stem bark, leaves, fruits, and seeds of the plant are used as medicines mainly in the form of decoction in various areas globally with varying applications [15]. In Mauritius, Ecuador, and Papua New Guinea, leafy preparations are applied locally to pain sites [8,16]. Furthermore, in Brazil, Mexico, Nicaragua, and Martinique leaf extract serves as an analgesic when ingested, and formulations are used in the management of flu, cold, and asthma in asthmatics [17,18]. Then in Malaysia, it is used to treat parasites both within and on the skin of humans [15]. In tropical countries, like Vietnam, Ghana, Cameroun, and Nigeria, leaf decoctions are employed in plasmodiasis treatment [17,18] as the mature ripe fruit serves as food in local societies around the world [12].
Pharmacological activities
Reviews on the pharmacological activities of the plant show that a greater percentage of the work has been on in vitro studies (about 66%), followed by in vivo (around 32%) and very little on clinical studies (within the 2% range). Extracts obtained for studies are mainly organic (about 80% - 90%) and less aqueous extracts (about 10%), which also mimics the way locals use the plants [8].
Phoenix dactylifera
Phoenix dactylifera L. commonly called date belongs to the Arecaceae or Palmae plant family, which comprises over 200 genera and about 2,500 species worldwide [19]. Phoenix is the genus grown because it has sweet edible fruits. P. dactylifera (date palm) is seen as one of the most ancient staple crops that have been grown since the time of old by humans as food in the Middle East, Southwest Asia, Saudi Arabia, and North Africa, especially in Egypt [19]. Additionally, the date fruit is grown in Australia, Latin American countries, South Africa, and the United States of America [20-23]. Dates can grow in very hot and dry climates and tolerate salty and alkaline soils relatively well with little rainfall and very low humidity [20]. The flowers of the date palm plant are small and yellowish and hang directly on the spikelets that develop into the date fruits. Fruits are berries with pits surrounded by fibrous paper endocarp, fleshy mesocarp, and fruit skin (pericarp). Trees in different continents globally produce fruits that vary in shape, size, weight, organoleptic, and physicochemical properties [20,24]. There are reports in the literature on some of the phytochemicals in the date fruit; these include tannins, sterols, flavonoids phenols, and carbohydrates [25,26].
Ethno medicinal uses
Several medicinal uses are connected to consuming Phoenix dactylifera L. fruit either directly or indirectly. The fruit has high tannin content, which makes it a good astringent for treating intestinal problems [20]. The fruit is formulated as decoctions; syrups, pastes, and infusions are often administered for colds, sore throats, and bronchial coughs [27]. The pulverized dried roots of the plant are useful in the treatment of toothache in regions where it is commonly located. Pollen from the palm contains the estrogenic compound, which aids fertility in women [28].
Pharmacological activities
Several pharmacological activities have been reported on Phoenix dactylifera L. which include antidiabetic, anti-tumor, anti-inflammatory, antimicrobial, nephroprotective, and antioxidant activities [19].
Pterocarpus osun
Pterocarpus osun L. is a deciduous leguminous plant belonging to the Fabaceae family. It is found in the savannah region of Africa and arid forests and is widespread in tropical regions worldwide [29]. The plant is commonly known by many as “camwood” and given different names by various tribes in Nigeria and wherever they may be found globally. P. osun L. has several common names including Vene in French, Bani or Banuhi in Burkina Faso, Kino in Gambia, Palissandre in Senegal, Osun-dudu in the Southwestern part of Nigeria, and Madubiya in Northern Nigeria. Besides the use of this plant in traditional medicine, many people use the wood because it serves as fuel in cooking by the locals and has made the plant an endangered species [29]. The plant serves as a natural dye locals use during cultural displays and also in some of their cosmetic preparations [6].
Phytochemicals that have been reported on Pterocarpus osun L. include flavonoids, tannins, terpenoids, alkaloids, glycosides, and phenols [6,29].
Ethno medicinal uses
The stem of P. osun when pulverized into fine particle size is administered to prevent conditions resulting from severed umbilical cords in newborns [30,31]. Use of the plant in the treatment of eczema, candidiasis, rheumatoid arthritis, gonorrhea, and acne has also been reported [31]. In addition, stem is formulated into herbal medicine, NICOSAN which is used for treating individuals with sickle cell condition and amenorrhoea [31]. Furthermore, the dried leaves are used to formulate black soap traditionally while the bark, heartwood, and roots are also formulated into skin care products for individuals [31]. The use of Pterocarpus spp in the treatment of type 2 diabetes mellitus has been reported [29].
Pharmacological activities
P. osun stembark extracts have shown good activity against Alcaligenes faecalis, Bacillus cereus, Enterobacter aerogenes, Escherichia coli, Proteus vulgaris, Pseudomonas aeruginosa, Bacillus subtilis, and Staphylococcus aureus. Other literature established that Pterocarpus species (P. cannabinoids, P. soyaxuii, and P. osun) exerted antimicrobial activities on different microorganisms [31-33].
Harungana madagascariensis
Harungana madagascariensis (Lam. ex. poir) is a flowering tree belonging to the Hypericaceae family [34]. It is distributed throughout tropical and subtropical African regions and is particularly native to Central Africa, the Republic of Congo, the Democratic Republic of Congo, Equatorial Guinea, Angola, Ethiopia, Lesotho, Sudan, and South Africa and also found in western African countries including Nigeria [35]. It is a small size bushy tree with a height of about 4 m - 7 m with fruits that turn red or yellow when fully mature [35,36].
Flavonoids, alkaloids, steroids/triterpenoids, lanthanoids, anthraquinones, tannins, phlorotannins, saponin, carbohydrates, and xanthones are some of the phytochemicals present in the plant [37,38].
Ethno medicinal uses
Decoction of the leaves and the stem bark are used locally in treating anemia, malaria, fever, gastrointestinal disorders, and nephrosis [37]. Additionally, root preparations are used in treating ailments such as hemorrhoids, leprosy, and gonorrhea [37]. Furthermore, the leaf preparations are used in treating dysentery and skin problems and for wound dressing by Ghanaians [36-39]. People of Sierra Leone use the leaves and bark to arrest post-partum hemorrhage and as medication for digestive problems [37,40,41].
Pharmacological activity
The local uses agree with the pharmacological in vitro and in vivo investigations of the plant extracts and the activities include antimicrobial, anti-protozoan, anti-sickling, and anti-inflammatory [42-44].
Rutidea parviflora DC.
Rutidea paviflora DC is of the Rubiaceae plant family. It is found throughout the regions of West Africa; Senegal, Guinea, Sierra Leone, Ivory Coast, Liberia, Equatorial Guinea, Ghana, and Nigeria. It is located around the South-West and South-Eastern regions of Nigeria. Flavonoids, tannins, alkaloids, saponins, terpenoids, carbohydrates, and cardiac glycosides are some phytochemicals reported [45].
Ethno medicinal uses
R. parviflora is reportedly used by the people of Delta state, Nigeria in treating different conditions including inflammation, cancers, convulsions, and other seizure types [45,46].
Pharmacological activity
In vitro, the pharmacological activity of the plant has been documented. Extracts of R. parviflora were active against four ovarian cancer cell lines [45].
Brine Shrimp (Artemia salina) Lethality Test (BSLT)
BSLT is a simple preliminary cytotoxicity assay for screening bioactive compounds and other chemical substances [47]. Some of the bioactive chemicals include plant extracts [48], cyanobacteria toxins [49], and other chemicals such as pesticides [50] and heavy metals [51]. Among these benchtop assays for determining the toxicity of substances; are yellow fever mosquito larvae lethality assay [52], frond proliferation inhibition in duckweed [53] and crown gall tumor inhibition on discs of potato tubers [54]. BSLT remains one of the tests that does not require sophistication and most cost-effective [55].
Allium cepa Cytotoxicity Assay (ACCA)
This assay is used to investigate the potential genotoxicity of a variety of chemical, physical, and biological agents. There is a long history in the scientific literature, from the first experiment carried out [56], followed by a more standardized examination method [57] and then recently [58,59].
Reports of some other plants that have been employed in cytotoxicity bioassay recently: Zea mays and Drimia indica (Roxb.) Jessop [60,61]. Despite the use of these plants, A. cepa has the advantage over them because of the large size of chromosomes that are seen under the light microscope [59].
The overall objective of this study is to evaluate the cytotoxic and genotoxic effects of the medicinal plants’ extracts using the Brine shrimp lethality test (BSLT) and Allium cepa cytotoxicity assay (ACCA) and to identify the bioactive chemical compounds in the extracts using GC-MS.
Materials
Collection, identification and authentication: Plant materials of Annona muricata (leaf), (herbarium No. UPHA0564); Phoenix dactylifera (pit), (Herbarium No. UPHA0565); Harungana madagascariensis (stembark), (Herbarium No. UPHH0566) Pterocarpus osun (heartwood), (Herbarium No. UPHH0567) and Rutidea paviflora (rootbark), (INTERCEDD/1588) were sourced from a bio-reserve in Delta State, Nigeria and authenticated by a botanist, Mr Alfred O. Ozioko (INTERCEDD) with expert advice offered by Prof. J. F. Bamidele of the Department of Plant Biology and Biotechnology, University of Benin, Nigeria. Voucher specimen numbers were deposited in the herbarium.
Equipment and Instruments: Photo microscope, Electronic weighing balance (model WT6002A), Grinding machine, Maceration jars, Thermostat bath (HH-6; Techmel and Techmel, USA), Lypholiser (Harvest right scientific freeze dryer), Beakers, Glass funnels, Measuring cylinders, Conical flask, Rotary evaporator (R-205), Desiccator, Spatula, Crucibles, Filter papers, Syringes and Digital thermometers, Gas Chromatography-Mass Spectrometer (GC-MS) (7890B GC system coupled with an Agilent 5977A MSD with a Zebron-5MS column (ZB-5MS 30 m × 0.25 mm × 0.025 μm) (5%-phenylmethylpolysiloxane).
Reagents: Methanol, Dichloromethane of Analytical grade (Sigma-Aldrich). Chloroform, Diethyl Ether, Acetic Anhydride, Glacial acetic acid, Sodium picrate, 2% 3,5-Dinitrobenzoic acid, Picric acid, Iodine solution, Dimethylsulfoxide, (JHD company, Guangdong. GuanghuaSci-Tech. Co. Ltd. China), Wagner’s reagent, Ammonia solution, Saturated lead acetate solution, Hydrochloric acid, Dragendorff’s reagent, Million’s reagent, Benedict’s solution, 7.5% Potassium Hydroxide, Sodium Hydroxide, Kedde reagent, Ferric chloride solution, Fehling’s solution A and B (Sigma Aldrich Chemicals, St Louis, USA), Deionized water, Distilled water (Pharmaceutical Chemistry Lab, University of Port Harcourt).
Methods
Extraction of the plant materials: The plant materials were extracted according to the American National Cancer Institute (NCI) method of extraction. 200 g of pulverized plant was macerated in a mixture of dichloromethane (500 ml) and methanol (500 ml) in 1:1 for 24 h. The plant material ratio to the solvent used was 1:5 and maintained for all weighed amounts of plant materials used. The obtained solution containing the extracts was decanted and methanol (500 ml) was added to the residue and allowed to stand for another 24 h. The solution of the extract was collected by filtration and 1000ml of deionized water was added to the residue. The aqueous extract was collected after 24 hours of maceration. The methanol extraction was combined with the 1:1 dichloromethane and methanol extraction to yield the organic extract. This extraction solution was evaporated to dryness on a rotary evaporator at a temperature of 40 ̊C. The obtained dry extracts were further dried in a desiccator to remove any trace of solvent. The aqueous extraction was dried using a lyophilizer to obtain a solid sample.
The percentage yields of the crude extracts of the plant were calculated as follows:
Phytochemical screening: The plant extract (crude extract of the stem bark) was subjected to preliminary analysis using the method described by Trease and Evans [62].
GC-MS analysis of the organic extracts: The gas chromatography-mass spectrometry (GC-MS) analysis of the DSE was quantitatively determined using an Agilent 7890B GC system coupled with an Agilent 5977A MSD with a Zebron-5MS column (ZB-5MS 30 m × 0.25 mm × 0.025 μm) (5%-phenylmethyl polysiloxane). The GC-grade helium served as the carrier gas at a constant flow rate of 2 mL/min. The DSE was dissolved with ethanol and filtered before use. The column temperature was maintained at 60 °C and gradually increased at 10 °C per minute until a final temperature of 300 °C was reached. The time taken for the GC-MS analysis was 30 min. The compounds were identified based on computer matching of the mass spectra with the NIST 11 MS library (National Institute of Standards and Technology Library).
Experimental design
This study was designed in line with the ethically approved experimental protocols adopted by the Department of Experimental Pharmacology and Toxicology, of the Faculty of Pharmaceutical Sciences, University of Port Harcourt.
Artemia salina lethality test
Brine shrimp (A. salina) eggs obtained from Thailand, (American Eagle brand, the Great Sea Lake, USA) were incubated in artificial seawater (ASW) in a hatching vessel at a temperature of 28 ± 2 ⁰C for 24 h after which the hatched nauplii were left for 24 h more in the same experimental conditions for further development to the meta nauplii stage.
10 nauplii of 48 h’ old were introduced into 10ml beakers containing concentrations of the sample done in triplicate for the six concentrations and control. ASW was then added to make the volume up to 1 ml. 1 ml of different concentrations of the plant extract; 10, 100, 250, 500, 750, and 1000 µg/ml was used to make volume up to 2 ml. They were allowed for 24 h at a temperature of 28 ± 2 ˚C with a sufficient amount of light in a well-aerated environment. ASW was used as the control and solubilizing agent for the experiment. 24 h later, the number of dead Artemia salina was counted, and recorded and the lethal concentration 50% (LC50) value and 95% confidence interval were analyzed using the probit analytical tool. Mortality percentage of the dead nauplii was calculated using the formula below
Allium cepa test
Onion bulbs (Allium cepa L., 2n = 16) of about 1.5 cm - 2.5 cm were purchased from a local market at Choba in Port Harcourt, Nigeria, were sundried for 2 wks, and the outer covering carefully taken off leaving the root primordial unscratched that were then used for the assay by the standard procedures [36].
Three onion bulbs were selected and used for the six concentrations (100, 250, 500, 750, 1000, and 2500 µg/ml) of the five plant extracts and also for the control (distilled water) at a pH of 7.3. The base of the onion bulbs was suspended in beakers containing samples for test and kept in the dark for 72 h while test samples were changed every 24 h and at 72 h exposure to the sample solution, measurements of A. cepa root length were taken using a meter rule for bulbs with very good growth in centimeters. The different concentrations of plant extracts and that of the control, the percentage of root growth inhibition about the negative control, and EC50 (effective concentration at which root growth equals 50% of the test control) for various plant extracts were obtained. Newly grown roots were cut from each onion bulb and examined for possible morphological abnormalities [63].
Evaluation of induction of chromosomal aberration, Onion bulbs (3) were placed in test tubes containing different concentrations (100, 250, 500, 750, 1000, and 2500 µg/ml) of plant extracts and the control for 72 h. Root tips of the onion bulbs were cut after 72 h, fixed in ethanol (3): glacial acetic acid (1) (v/v) and hydrolyzed in 1N HCl at a temperature of 60 ⁰C for 5 mins and washed in distilled H2O.
The tips of two roots were crushed on each slide, and stained with acetocarmine dye for 10 mins and cover slips were carefully placed to eliminate the entrance of the air bubble. Clear fingernail polish was used to seal the cover slips on slide [64] with little modifications. Slides were prepared for various concentrations (6 slides) and that of the control (1 slide) making the total number of slides up to 7, cells per slide were examined using a microscope with magnification of x1000.
The mitotic index (MI) was calculated using the equation below [65].
Measurement of the A. cepa root length was used as the index of general toxicity after 72 h of exposure to different concentrations of plant extracts.
Statistical analysis
The BLST and Allium cepa assay were done in triplicates. The data were presented as mean ± standard error of the mean (SEM). The significant differences of the five extracts were found using the Two-way analysis (ANOVA), with Monte Carlo posthoc test. A p value of less than 0.05 was deemed significant. The program Graph Pad Prism version 8.0 (GraphPad Software, LA Jolla, CA, USA) was used for all statistical analysis.
Table 1 illustrates the yield of the five medicinal plants after the pulverized plant materials were macerated in dichloromethane and methanol first in a ratio of 1:1. Marc was later macerated in methanol, filtered and placed in the desiccator for a few days to remove any remaining solvent. Weights of the dry extracts were taken and later placed back in the desiccator, after 2 h weights were checked until they were constant. High yield was obtained from A. muricata leaves compared to the amount obtained from other plants that were macerated.
| Table 1: Percentage yield of Plants’ crude extract. | |
| Plant Extract | Percentage Yield(%W/W) |
| Annona muricata | 9.0 |
| Harungana madagascariensis Lam | 5.7 |
| Phoenix dactylifera L. | 8.5 |
| Pterocarpus osun L. | 9.8 |
| Rutidea paviflora | 2.5 |
Table 2 illustrates the phytochemical screening result of the five medicinal plants namely, Annona muricata, Harungana madagascariensis, Phoenix dactylifera, Pterocarpus osun, and Rutidea parviflora.
| Table 2: Phytochemical Screening of Plants’ extracts (crude/organic) | ||||||
| Test Type | Annona muricata | Harungana mascariensis | Phoenix dactylifera | Pterocarpus osun | Rutidea parviflora | |
| 1 (a) (b) (c) |
Alkaloids Dragendorff’s Test Meyer’s Test Hager’s Test |
- + - |
+ - - |
+ - + |
+ - - |
- + + |
| 2 (a) (b) |
Flavonoids Shinoda Test Aluminum Chloride Test |
+ + |
+ + |
+ - |
+ - |
- + |
| 3 (a) |
Tannins Ferric chloride Test |
+ |
+ | + | + | + |
| 4 (a) (b) |
Carbohydrates MolishTest Fehling’s Test |
+ - |
- - |
- + |
+ - |
- + |
| 5 (a) (b) |
Saponins Frothing Test Emulsion Test |
- + |
- - |
+ + |
+ - |
+ + |
| 6 (a) |
Phlobatannins Hydrochloride acid Test |
+ |
+ | + | + | - |
| 7 (a) (b) |
C-Glycosides Keller killiani Test Kedde Test |
+ + |
+ + |
- - |
+ + |
+ + |
| 8 (a) (b) |
Triterpenoids/Steroids Leibermann-Buchard Test Salkwoski test |
+ + |
+ + |
+ + |
- - |
+ + |
| 9 (a) |
Anthraquinone Bontrager Test |
- | - | + | + | - |
| 10 (a) |
Phenol Ferric Chloride Test |
+ | - | + | + | + |
| Key: (+) = Present (-) = Absent | ||||||
A. muricata leaves showed the presence of alkaloids, flavonoids, saponins, phlorotannins, terpenoids, carbo-hydrates, and cardiac glycosides. Phytochemicals obtained are in order with previous research work [4,8,14].
Harungana madagascariensis phytochemicals [14,37] agree with those in this research work and include alkaloids, tannins, flavonoids, phlorotannins, steroids/triterpenoids, carbohydrates, and saponins.
Reports obtained from other work include tannins, steroids, flavonoids, phenols, and carbohydrates in Phoenix dactylifera [1,19,26]. However, saponins and anthraquinones were also observed in addition to those mentioned earlier.
Flavonoids, tannins, terpenoids, alkaloids, glycosides, and phenols are reported phytochemicals in Pterocarpus osun [6,9]. Phytochemicals obtained correspond to existing literature with the current work showing the presence of saponins, phlobatannins, and anthraquinones.
Rutidea parviflora DC has been reported to contain phytochemical compounds which include flavonoids, tannins, alkaloids, saponins, terpenoids, carbohydrates, and cardiac glycosides [45] and they are following the result obtained.
The results of the BSLT assay on the five medicinal plants are contained in Table 3. The findings showed that the mortality was dose-dependent, with 1000 µg/ml concentration resulting in high fatality or total elimination of the nauplii.
| Table 3: Mortality in percent of the five medicinal plants’ extracts on Artemia salina after 24 h of exposure in the Brine shrimp lethality test (BSLT). | |||||
| Concentration of plant extract (µg/ml) | % mortality of Brine shrimp after 24 h (Mean ± SEM) | ||||
| H. madagascariensis | P. osun | P. dactylifera | A. muricata | R. parviflora | |
| 10 | 17.0 ± 0.2 | 17.0 ± 0.2 | 7.0 ± 0.2 | 7.0 ± 0.2 | 13.0 ± 0.2 |
| 100 | 43.0 ± 0.2 | 30.0 ± 0.2 | 17.0 ± 0.2 | 17.0 ± 0.2 | 23.0 ± 0.2 |
| 250 | 53.0 ± 0.2 | 50.0 ± 0.2 | 27.0 ± 0.2 | 27.0 ± 0.2 | 37.0 ± 0.2 |
| 500 | 63.0 ± 0.2 | 100.0 ± 0.0 | 43.0 ± 0.2 | 43.0 ± 0.2 | 43.0 ± 0.2 |
| 750 | 77.0 ± 0.2 | 100.0 ± 0.0 | 53.0 ± 0.2 | 53.0 ± 0.2 | 50.0 ± 0.2 |
| 1000 | 93.0 ± 0.2 | 100.0 ± 0.0 | 73.0 ± 0.2 | 73.0 ± 0.2 | 53.0 ± 0.2 |
Table 4 shows the percentage mortality and LC50 values for all the plants in the BSLT.
| Table 4: LC50 values of the five plants’ extracts in BSLT. | |
| Plant | LC50 (µg/ml) |
| Harungana madagascariensis | 235.9 |
| Pterocarpus osun | 250.0 |
| Phoenix dactylifera | 660.7 |
| Annona muricata | 660.7 |
| Rutidea parviflora | 750.0 |
In this test H. madagascariensis, Pterocarpus osun, Phoenix dactylifera, Annona muricata and Rutidea parviflora had LC50 values of 235.9, 250.0, 660.7, 660.7 and 750.0 µg/ml respectively. H. madagascariensis was the plant with the least LC50 which implies that the dose that was lethal on 50% of the Brine shrimp population was smaller than the other plants. However, for Pterocarpus osun when the concentration increased to 500 µg/ml it was lethal to the whole Brine shrimp population in the test medium. Phoenix dactylifera and Annona muricata had similar LC50 values in the experiment while that of Rutidea parviflora extract was 750.00 µg/ml which was the least toxic of the five plants.
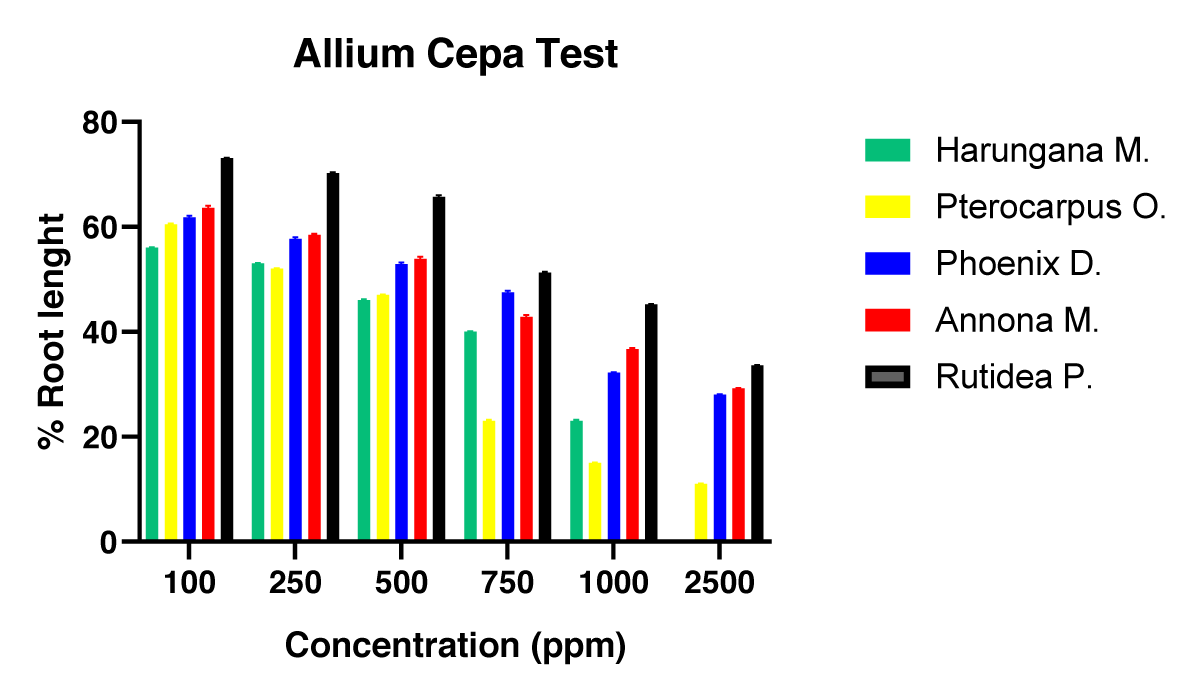
Further evaluation of the cytotoxicity of these plants was demonstrated on the Allium cepa test, in which the root tips of the onion bulbs were exposed to the solutions of the different plant extracts at various concentrations. The obtained results are portrayed in Figure 1.
Figure 1: Effect of the five plants’ extracts on cell division of A. cepa root after 72h of exposure in the cytotoxicity test (ACCA).
Again, a dose-dependent cytotoxicity is observed and the most cytotoxic of the five medicinal plants was Harungana madagascariensis. While the least cytotoxic of these plants was Rutidea parviflora. The EC50 of the extracts was determined and is presented in Figure 2.
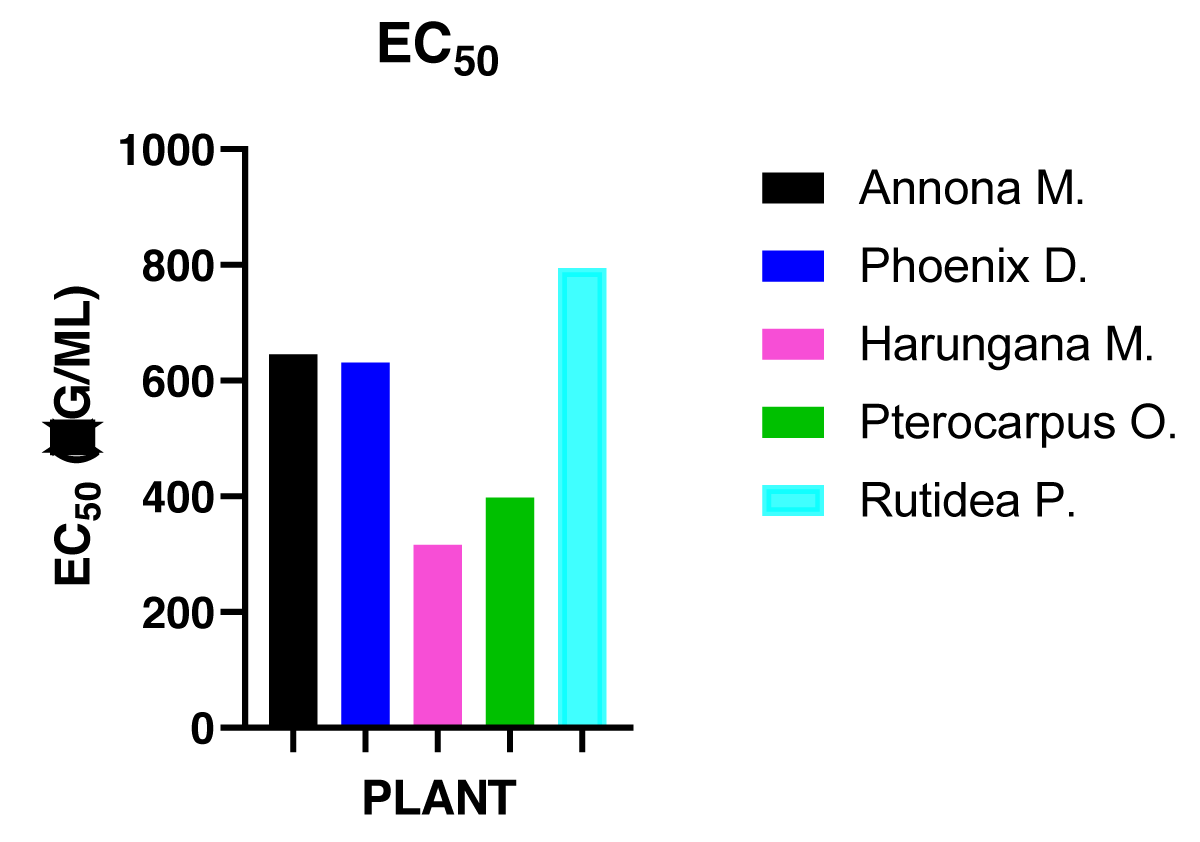
Figure 2: ACCA EC50 (µg/ml) values for the effect of plants’ extracts on cell division of A. cepa root.
Different plants’ extracts had an inhibitory effect on the A. cepa root growth [66-68]. After 72h of exposing the growing onion bulbs to different concentrations of various plant extracts, some had a very good inhibitory effect on the dividing plant cells of which Harungana madagascariensis was the most effective. Root tips of the onion bulbs were measured for each concentration, and a plot was made to obtain the EC50 as shown in Figure 2. Harungana madagascariensis had 316.23 µg/ml and the least was R. parviflora. A concentration-dependent inhibition was observed which was also seen with the root number in Figure 1 and is in line with previous work [57,63,69,70].
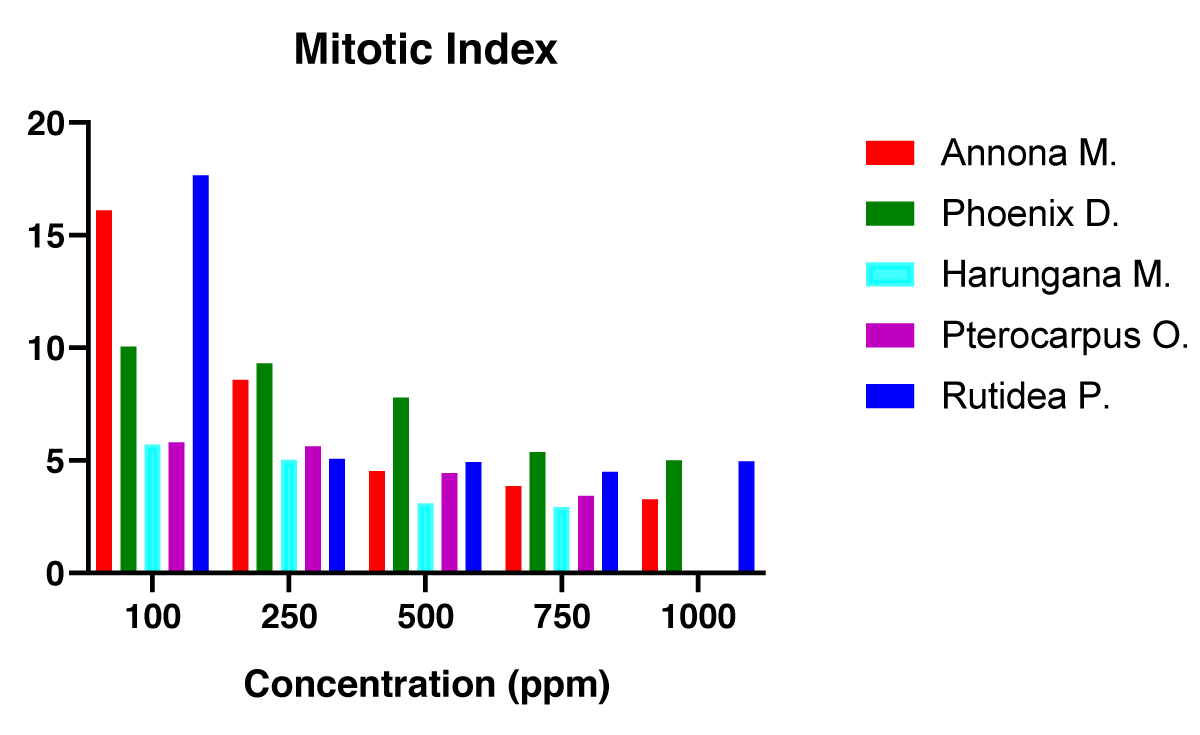
The Mitotic Index of the onions treated with different concentrations of the five medicinal plants was determined and the results displayed in Figure 3.
Figure 3: The Mitotic index of the onion cells treated with the extracts at different concentrations. The percentage of aberrant cells was determined for the plant extracts and presented in Figure 4.
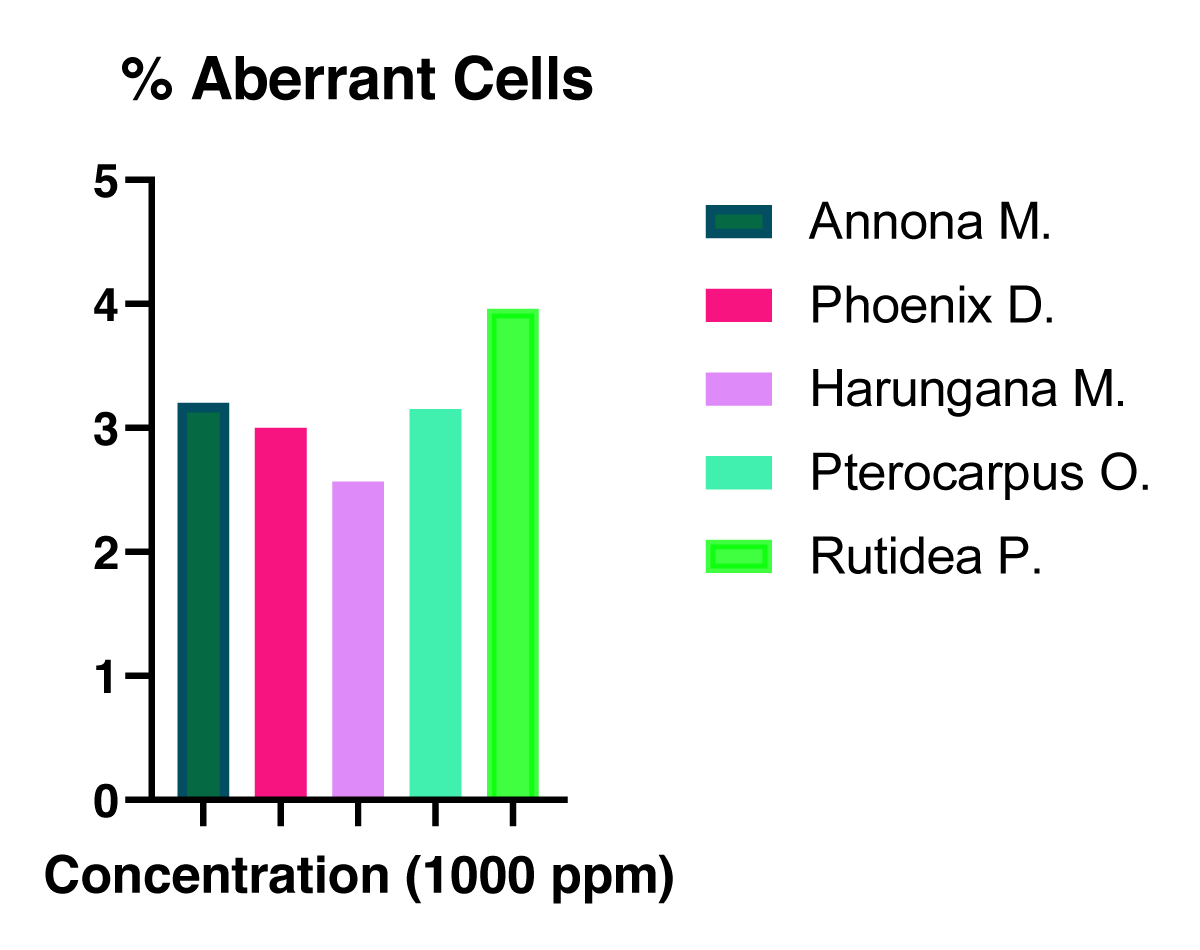
The percentage of aberrant cells was determined for the plant extracts and presented in Figure 4.
Figure 4: The % Aberrant cells in the number of dividing cells of the plant extracts-treated onion bulbs at a concentration of 1000 ppm.
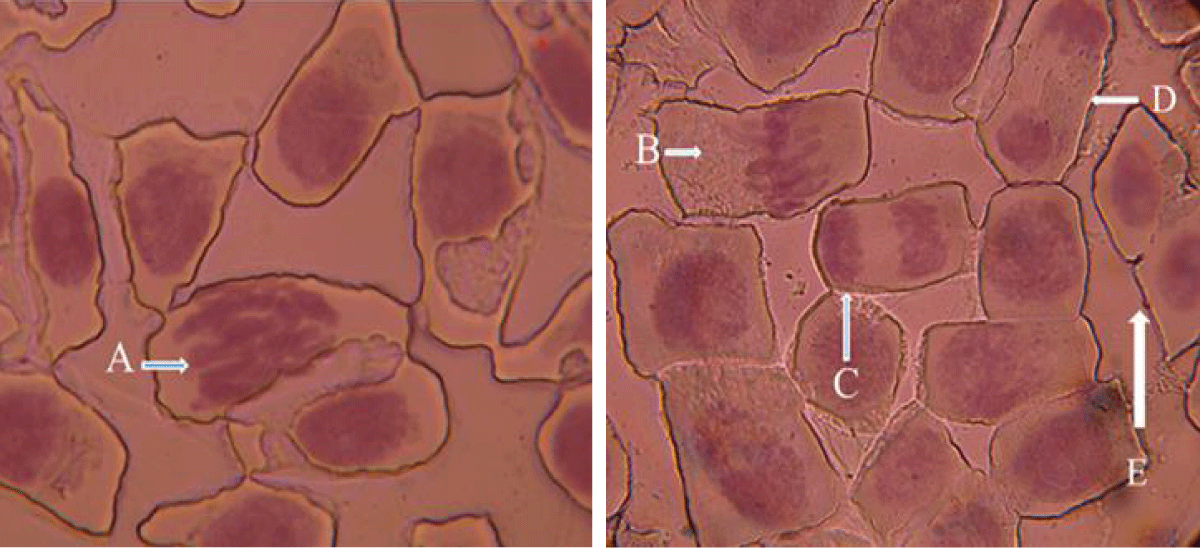
The different stages of the mitotic division in the Allium cepa root tips are shown in Figure 5.
Figure 5: Photograph of squashed A. cepa root tip showing the stages of mitosis in ACCA. A = Prophase, B = Metaphase, C = Anaphase, D = Telophase, E = Interphase.
The results contained in Figure 3 revealed that Harungana madagascariensis had the least MI, which by implication, indicates lesser cell division occurred in the root tips of the Allium cepa. treated with the different concentrations of this plant. Thus, the findings from the mitotic index (MI), showed that more toxic plant extracts had lower MI, while less toxic plant extracts had higher MI.
Again the % aberrant cells present in the 1000 ppm extract-treated root tips of the onion bulbs as presented in Table 4 above, demonstrated that there was no significant variation in the aberrant cells population.
The GC-MS analysis of the five investigated medicinal plants was carried out to determine the non-polar and moderately polar potential bioactive compounds and cytotoxic compounds present in these plants. A literature search was further carried out to identify the documented activities of the identified compounds. The GC-MS results are shown in Tables 5-9.
| Table 5: GC-MS Analysis of P. osun Heartwood Organic Extract. | ||||||
| S/N | RT (Min) | Peak Area (%) | MW | MF | Compound | Documented Bioactivity |
| 1 | 9.233 | 12.12 | 294 | C19H34O2 | 9,12-Octadecadienoic acid (Z, Z)-, methyl ester | Antioxidant [71] Antimicrobial [72] Anticancer [71] |
| 2 | 9.279 | 19.23 | 296 | C19H36O2 | 6-Octadecenoic acid, methyl ester,(Z)- | Antipyretic [73] |
| 3 | 9.68 | 1.11 | 294 | C19H34O2 | 9,15-Octadecadienoic acid, methyl ester, (Z, Z)- | Anti-cancer and anti-inflammatory [74]. |
| 4 | 16.25 | 39.57 | 284 | C17H16O4 | Homopterocarpin | Cytotoxic to human cancer cells [75] |
| 5 | 16.065 | 3.05 | 284 | C17H14O5 | Pterocarpin | Cytotoxic to human cancer cells [75] |
| 6 | 20.93 | 0.94 | 286 | C17H18O4 | Phenol, 2-(3,4-dihydro-7-methoxy-2H-1-benzopyran-3-yl)-5-methoxy | Antioxidant [76] |
| Table 6: GC-MS Analysis of P. dactylifera pits organic extract. | ||||||
| S/N | RT (Mins) | Peak Area (%) | MW | MF | Compound | Documented Bioactivity |
| 1 | 5.096 | 7.73 | 134 | C10H14 | 1,3,8-p-Menthatriene | Antimicrobial [77] |
| 2 | 16.185 | 9.38 | 270 | C17H34O2 | Hexadecanoic acid, methyl ester (methyl palmitate) | Antifungal, antioxidant antimicrobial, hypocholesterolemic, nematicidal, pesticidal, antiandrogenic flavor, hemolytic, 5-Alpha reductase inhibitor [78,79]. |
| 3 | 17.850 | 9.51 | 294 | C19H34O2 | 9,12-Octadecadienoic acid, methyl ester (oleic acid derivative) | Antibacterial, Antifungal [71,72] |
| 4 | 18.142 | 4.25 | 298 | C19H38O2 | Methyl stearate | Antifungal and Antioxidant [78] |
| 5 | 18.328 | 2.14 | 298 | C18H31C10 | 9,12-Octadecadienoyl chloride, (Z, Z)- | Antimicrobial [80] |
| Table 7: GC-MS Analysis of H. madagascariensis stem bark organic extract. | ||||||
| Peak | RT | Area (%) | MW | MF | Compound | Documented Bioactivity |
| 1 | 4.495 | 14.03 | 136 | C10H16 | β-Pinene | Antimicrobial, Antibacterial [81] |
| 2 | 5.119 | 134 | C10H14 | 1,3,8-p-Menthatriene | Antimicrobial [77] | |
| 3 | 6.509 | 0.18 | 154 | C10H18O | Terpinen-4-ol | Antifungal [82] |
| 4 | 7.333 | 1.28 | 154 | C10H18O | L-α-Terpineol | Anticancer, antioxidant [83] |
| 5 | 16.58 | 8.39 | 270 | C17H34O2 | Hexadecanoic acid, methyl ester | Antifungal, antioxidant, antimicrobial, hypocholesterolemic, nematicidal, pesticidal, antiandrogenic, flavor, hemolytic, 5-Alpha reductase inhibitor [78,79] |
| 6 | 17.163 | 0.18 | 294 | C17H34O2 | 9,12-Octadecadienoic acid, methyl ester | Antibacterial and Antifungal [71,72] |
| 7 | 18.033 | 0.90 | 298 | C19H38O2 | Methyl stearate | Antifungal and Antioxidant [78] |
| Table 8: GC-MS Analysis of A. muricata leaves organic extract | ||||||
| S/N | RT (Mins) | Peak Area (%) | MW | MF | Compound | Documented Bioactivity |
| 1 | 15.264 | 0.11 | 296 | C20H40O | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | Antibacterial, Antioxidant [84] |
| 2 | 16.162 | 0.71 | 270 | C17H34O2 | Hexadecanoic acid, methyl ester | Antifungal, antioxidant, antimicrobial, hypocholesterolemic, nematicidal, pesticidal, antiandrogenic, flavor, hemolytic, 5-Alpha reductase inhibitor [78,79] |
| 3 | 17.822 | 0.56 | 294 | C17H34O2 | 9,12-Octadecadienoic acid, methyl ester | Antibacterial, Antifungal [71,72] |
| 4 | 18.142 | 0.32 | 298 | C19H38O2 | Methyl stearate | Antifungal and Antioxidant [78] |
| 5 | 20.74 | 1.78 | 312 | C20H40O2 | Octadecanoic acid, 11-methyl-, methyl ester | Antimicrobial [72] |
| Table 9: GC-MS Analysis of R. parviflora root-bark organic extract. | ||||||
| S/N | RT(Mins) | Peak Area % | MW | MF | Name of Compound | Documented Bioactivity |
| 1 | 8.455 | 0.82 | 218 | C15H22O | 2(1H)Naphthalenone, 3,5,6,7,8,8a-hexahydro-4,8a-dimethyl-6-(1-methylethenyl)- | Tyrosinase Inhibitor [7] |
| 2 | 9.765 | 0.19 | 280 | C20H40 | Cyclohexane, 1-(1,5-dimethylhexyl)-4-(4-methylpentyl)- | Antimicrobial activity Antibacterial and Anti-cancer [85] |
| 3 | 10.869 | 3.19 | 282 | C18H34O2 | cis-Vaccenic acid |
Antibacterial, Hypolipidemia [86] |
| 4 | 11.075 | 9.10 | 284 | C18H36O2 | Octadecanoic acid |
Antifungal, Antitumor, Antibacterial [72] |
| 5 | 13.038 | 0.70 | 286 | C17H34O3 | Hexadecanoic acid, 10-hydroxy-, methyl ester | Antifungal, Antioxidant, Antimicrobial, Hypocholesterolemic [78] |
| 6 | 15.973 | 0.23 | 330 | C19H38O4 | Octadecanoic acid, 9,10-dihydroxy-, methyl ester | Antibacterial and Antifungal [72] |
| 7 | 36.841 | 2.51 | 412 | C29H48O | Chondrillasterol | Cytotoxic [72] |
Table 5 shows the GC-MS analysis for P. osun heartwood organic extract. The information on the chromatogram of the sample is represented as retention time (RT), peak area (%), molecular weight, molecular formula, name of the compound, and documented bioactivity. Some of the compounds seen are; 9,12-Octadecadienoic acid (Z, Z)- methyl ester, 6-Octadecenoic acid (Z)- methyl ester, hemopterocarpin, and pterocarpin. These compounds have been shown to possess various bioactivities including antimicrobial, antipyretic, anti-inflammatory, and cytotoxic activity. The toxicity effects of P. osun extract on Artemia salina and Allium cepa could be attributed to the homopterocarpin and pterocarpin compounds identified by the GC-MS analysis. The plant was one of those that exhibited a great level of toxicity on A. salina and A. cepa as observed in the study.
Table 6 shows the result of the GC-MS analysis of Phoenix dactylifera pits extract represented as the peak, retention time, area (%), molecular weight, molecular formula, compound, and documented bioactivity. The chromatogram showed peaks and some of the compounds are displayed in the Table. 1,3,8-p-menthatriene, hexadecanoic acid, methyl ester (methyl palmitate), and 9,12-Octadecadienoic acid, methyl ester (oleic acid derivative) are some of the compounds. Most of these compounds have been reported to have antimicrobial and antioxidant activities. Phoenix dactylifera pits extract exhibited a good toxic effect in the research and looking at the nematicidal and pesticidal activities of hexadecanoic acid, methyl ester (methyl palmitate) in Table 8, this probably points to the lethal effect on Brine shrimp and A. cepa in the research.
Table 7 shows the GC-MS analysis for H. madagascariensis stembark organic extract. Compounds represented in the Table are; β-Pinene, 1,3,8-p-Menthatriene, Terpinen-4-ol, L-α-Terpineol, and hexadecanoic acid methyl ester. Most of the compounds have documented antimicrobial activity. Also, hexadecanoic acid methyl ester has nematicidal and pesticidal activities which probably could be responsible for the high toxicity effect seen on both brine shrimp and Allium cepa in the study. Furthermore, the documented anticancer activity of L-α-Terpineol could also be suggestive of the toxic effects observed in the research.
Table 8 represents the GC-MS analysis for the organic leaf extract of A. muricata. Compounds are 3,7,11,15-Tetramethyl-2-hexadecen-1-ol, hexadecanoic acid methyl ester, and 9, 12- Octadecadienoic acid methyl ester. These are compounds with documented antibacterial, antioxidant, and antifungal bioactivities. However, the recorded nematicidal and pesticidal activities of the plant as shown in the chromatogram could be responsible for the toxicity effect on Brine shrimp and A. cepa.
Table 9 shows the GC-MS analysis of R. parviflora root-bark organic extract. Compounds are 2(1H) Naphthalenone, 3,5,6,7,8,8a-hexahydro-4,8a-dimethyl-6-(1-methylethenyl)-, Hexadecanoic acid, 10-hydroxy-methyl ester. Activities such as antifungal, antimicrobial, antineoplastic, and cytotoxicity have been reported. The experiment carried out showed the plant to be toxic to the Brine shrimp and A. cepa, although it was not as other plants however, it exhibited toxicity.
The study of the cytotoxicity of the five selected medicinal plants was carried out in this research. The literature survey conducted showed that these plants had a rich ethnopharmacological history of use in numerous societies. It was discovered that plants like R. parviflora, and P. osun had a paucity of documented activities.
The phytochemical screening undertaken in this study documented that the main classes of phytoconstituents present in these plants are alkaloids, tannins, terpenoids, and saponins. These classes of compounds have been linked to several biological activities. The GC-MS analysis showed that hexadecanoic acid, methyl ester (methyl palmitate), and 9,12-Octadecadienoic acid, methyl ester (oleic acid derivative) are some of the compounds of the abundant compounds present in these plants, as it cuts across the five plants. These compounds are fatty acids with documented bioactive properties.
In terms of the cytotoxic propensity of the five selected medicinal plants, it could be said that the contributions of hexadecanoic acid, methyl ester, and 9,12-Octadecadienoic acid, methyl ester are negligible, as these compounds were predominantly present in all the extracts.
The cytotoxicity of P. osun. is most likely attributable to the presence of hemopterocarpin and pterocarpin. These compounds have been shown to possess various bioactivities including antimicrobial, antipyretic, anti-inflammatory, and cytotoxic activity [75,76]. The cytotoxicity of Harungana madagascariensis may be connected to the presence of L-α-Terpineol observed in the GC-MS analysis of its extract. The documented anticancer activity of L-α-Terpineol [83] could also be suggestive of the toxic effects observed in the research. The LC50 values for all the plants assayed in the BSLT showed significant differences. In this test Harungana madagascariensis, Pterocarpus osun, Phoenix dactylifera, Annona muricata and Rutidea parviflora had LC50 values of 235.9, 250.0, 660.7, 660.7 and 750.0 µg/ml respectively. The difference between the LC50 of Harungana madagascariensis and Pterocarpus osun was not significant, while the difference between both plants and the other three plants was significant at p < 0.001. Similarly, the inhibitory effect on the dividing plant cells was evaluated on the Allium cepa test. The EC50 of the five plant extracts was obtained. The values were 316.2, 398.1, 630.9, 645.6 and 794.3 respectively for Harungana madagascariensis, Pterocarpus osun, Phoenix dactylifera, Annona muricata and Rutidea parviflora. Again, a statistically significant difference was achieved at p < 0.001 for Harungana madagascariensis and Pterocarpus osun in comparison with Phoenix dactylifera, Annona muricata, and Rutidea parviflora. The Mitotic index obtained was also in consonance with the trend observed in both the BSLT and Allium cepa assays respectively. The order of the cytotoxicity of the five plants showed that cytotoxicity decreased from Harungana madagascariensis> Pterocarpus osun> Phoenix dactylifera> Annoma murocata> Rutidea parviflora on the Brine shrimp lethality test and Allium cepa cytotoxicity assay.
This study evaluated the toxicity of five medicinal plants; Harungana madagascariensis, Phoenix dactylifera, Pterocarpus osun, Annoma murocata, and Rutidea parviflora on the Brine shrimp lethality test and Allium cepa cytotoxicity assay. Both tests established that H. madagascariensis was the most toxic of the plants followed by P. osun, P. dactylifera, A. muricata, and lastly R. parviflora.
The phytochemical screening shows that the plants contain alkaloids, anthraquinone, flavonoids, tannins, saponins, triterpenoids/steroids, and phlabathanins
The brine shrimp lethality test and Allium cepa cytotoxicity assay showed that the plants’ extracts are moderately toxic which fall between 100 to 1000 µg/ml. Thus, these plants have a good safety profile and thus warrant further studies.
Limitations of the study
This study was carried out on only five medicinal plants representing five plant families that have demonstrated pharmacological prowess as shown by the numerous ethno-pharmacological and bioactivities documented on these plant families. Also, the cytotoxicity of the selected medicinal plants was assessed by only two cytotoxicity assays.
Recommendations
Given the resurgence of alternative medicine on the global scene, the cytotoxicity of medicinal plants employed for therapeutic purposes must be investigated. It is hoped that from this work, further evaluation of the safety of these plants such as sub-chronic toxicity investigations be carried out.
- Lombardi VR, Carrera I, Cacabelos R. In Vitro Screening for Cytotoxic Activity of Herbal Extracts. Evid Based Complement Alternat Med. 2017;2017:2675631. doi: 10.1155/2017/2675631. Epub 2017 Mar 13. PMID: 28386288; PMCID: PMC5366791.
- Hussain I, Ullah R, Ullah R, Khurram M, Ullah N, Baseer A, Khan FA, Khattak MR, Zahoor M, Khan J, khan N. Phytochemical analysis of selected medicinal plants. African Journal of Biotechnology. 2011; 10(38):7487-7492.
- Dar RA, Shahnawaz M, Qazi PH. General overview of medicinal plants: A review. 2017; 6(6):349–351.
- Agu KC, Okolie PN. Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata (Soursop). Food Sci Nutr. 2017 Jun 29;5(5):1029-1036. doi: 10.1002/fsn3.498. PMID: 28948021; PMCID: PMC5608983.
- Gulumian M, Yahaya ES, Steenkamp V. African Herbal Remedies with Antioxidant Activity: A Potential Resource Base for Wound Treatment. Evid Based Complement Alternat Med. 2018 Nov 22;2018:4089541. doi: 10.1155/2018/4089541. PMID: 30595712; PMCID: PMC6282146.
- Avwioro OG, Aloamaka PC, Ojianya NU, Oduola T, Ekpo EO. Extracts of Pterocarpus osun as a histological stain for collagen fibres. African Journal of Biotechnology. 2005; 4(5):460-462.
- Biswas R, Mukherjee PK, Dalai MK, Mandal PK, Nag M. Tyrosinase inhibitory potential of purpurin in Rubia cordifolia—A bioactivity guided approach. Industrial crops and products. 2015; 74:319-326.
- Coria-Téllez AV, Montalvo-Gónzalez E, Yahia EM, Obledo-Vázquez EN. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arabian Journal of Chemistry. 2018; 11(5):662–691.
- Rady I, Bloch MB, Chamcheu RN, Banang Mbeumi S, Anwar MR, Mohamed H, Babatunde AS, Kuiate JR, Noubissi FK, El Sayed KA, Whitfield GK, Chamcheu JC. Anticancer Properties of Graviola (Annona muricata): A Comprehensive Mechanistic Review. Oxid Med Cell Longev. 2018 Jul 30;2018:1826170. doi: 10.1155/2018/1826170. PMID: 30151067; PMCID: PMC6091294.
- Ribeiro de Souza EB, da Silva RR, Afonso S, Scarminio IS. Enhanced extraction yields and mobile phase separations by solvent mixtures for the analysis of metabolites in Annona muricata L. leaves. J Sep Sci. 2009 Dec;32(23-24):4176-85. doi: 10.1002/jssc.200900375. PMID: 19882621.
- Chatrou LW, Erkens RHJ, Richardson JE, Saunders RMK, Fay MF. The natural history of Annonaceae. Botanical Journal of the Linnean Society. 2012; 169(1):1–4.
- Moghadamtousi SZ, Fadaeinasab M, Nikzad S, Mohan G, Ali HM, Kadir HA. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int J Mol Sci. 2015 Jul 10;16(7):15625-58. doi: 10.3390/ijms160715625. PMID: 26184167; PMCID: PMC4519917.
- Frausin G, Lima RBS, Hidalgo AF, Maas P, Pohlit AM. Plantas da familia annonaceae tradicionalmente usadas como antimaláricos: Uma revisão. Revista Brasileira de Fruticultura, 36(SPEC. EDITION 1). 2014; 315–337.
- Natsir HH, Soekamto NH. J. Phys. 2019; 32027.
- Badrie N, Schauss AG. Soursop (Annona muricata L.): Composition, nutritional value, medicinal uses, and toxicology. In Bioactive Foods in Promoting Health. 2010; 621–643.
- World Health Organisation. Plant Medicinal in Papua New Guinea World Health Organization press, Manila. 2008; 26–27.
- Longuefosse JL, Nossin E. Medical ethnobotany survey in Martinique. J Ethnopharmacol. 1996 Sep;53(3):117-42. doi: 10.1016/0378-8741(96)01425-0. PMID: 8887020.
- Ross IA. Medicinal plants of the world, volume 3: Chemical constituents, traditional and modern medicinal. Springer Science & Business Media. 2007; 3.
- Rahmani AH, Aly SM, Ali H, Babiker AY, Srikar S, Khan AA. Therapeutic effects of date fruits (Phoenix dactylifera) in the prevention of diseases via modulation of anti-inflammatory, anti-oxidant and anti-tumour activity. Int J Clin Exp Med. 2014 Mar 15;7(3):483-91. PMID: 24753740; PMCID: PMC3992385.
- Chao CCT, Krueger RR. The date palm (Phoenix dactylifera L.): Overview of biology, uses, and cultivation. HortScience. 2007; 42(5):1077–1082.
- Al-Harrasi A, Rehman NU, Hussain J, Khan AL, Al-Rawahi A, Gilani SA, Al-Broumi M, Ali L. Nutritional assessment and antioxidant analysis of 22 date palm (Phoenix dactylifera) varieties growing in Sultanate of Oman. Asian Pac J Trop Med. 2014 Sep;7S1:S591-8. doi: 10.1016/S1995-7645(14)60294-7. PMID: 25312188.
- Al-Alawi RA, Al-Mashiqri JH, Al-Nadabi MJS, Al-Shihi BI. Date Palm Tree (Phoenix dactylifera L.): Natural Products and Therapeutic Options. Frontiers in Plant Science | Www.Frontiers in. Org. 2017; 8:845.
- Hazzouri KM, Flowers JM, Visser HJ, Khierallah HSM, Rosas U, Pham GM, Meyer RS, Johansen CK, Fresquez ZA, Masmoudi K, Haider N, El Kadri N, Idaghdour Y, Malek JA, Thirkhill D, Markhand GS, Krueger RR, Zaid A, Purugganan MD. Whole genome re-sequencing of date palms yields insights into diversification of a fruit tree crop. Nat Commun. 2015 Nov 9;6:8824. doi: 10.1038/ncomms9824. PMID: 26549859; PMCID: PMC4667612.
- Barghini P, Di Gioia D, Fava F, Ruzzi M. Vanillin production using metabolically engineered Escherichia coli under non-growing conditions. Microb Cell Fact. 2007 Apr 16;6:13. doi: 10.1186/1475-2859-6-13. PMID: 17437627; PMCID: PMC1857700.
- Martín-Sánchez AM, Cherif S, Ben-Abda J, Barber-Vallés X, Pérez-Álvarez JÁ, Sayas-Barberá E. Phytochemicals in date co-products and their antioxidant activity. Food Chem. 2014 Sep 1;158:513-20. doi: 10.1016/j.foodchem.2014.02.172. Epub 2014 Mar 12. PMID: 24731377.
- Ahmed A, Bano N, Tayyab M. Phytochemical and Therapeutic Evaluation of Date (Phoenix dactylifera). A Review Phytochemical and Therapeutic Evaluation of Date (Phoenix dactylifera). 2016; 11–17.
- Glasner B, Botes A, Zaid A, Emmens J. Date harvesting, packinghouse management and marketing aspects. In: A. Zaid (ed.). Date palm cultivation. Food and Agriculture Organization Plant Production and Protection paper no. 156. Food and Agri Culture Organization of the United Nations, Rome, Italy. 2002; 177–208.
- Hadrami ElA, Al-Khayri JM. Socioeconomic and traditional importance of date palm. Emirates Journal of Food and Agriculture. 2012; 24(5):371–385.
- Shobayo BI, Ojo DA, Agboola DA. Antibacterial Activity of Pterocarpus osun L. on Multi-Drug Resistant (MDR) Escherichia coli from Wound Infections in Abeokuta, South-West Nigeria. OALib. 2015; 02(08):1-6.
- Gill LS. Ethno- medical uses of plants in Nigeria, University of Benin Press, Nigeria. 1992; 276.
- Abayomi O, Olakunle F, Simon O, Fausat A, Sunday T. Comparative Studies of Chemical Composition of the Leaf Extracts of Pterocarpus osun from Different Geographical Regions of Nigeria. American Journal of Ethnomedicine. 2015; 2(5):2348–9502.
- Osuagwu G, Akomas C. Antimicrobial activity of the leaves of three species of Nigerian Pterocarpus (Jacq.). International Journal of Medicinal and Aromatic Plants. 2013; 3:178-183.
- Ebi GC, Ofoefule SI. Antimicrobial activity of Pterocarpus osun stems. Fitoterapia. 2000 Aug;71(4):433-5. doi: 10.1016/s0367-326x(00)00130-1. PMID: 10925018.
- Ndjakou Lenta B, Ngouela S, Fekam Boyom F, Tantangmo F, Feuya Tchouya GR, Tsamo E, Gut J, Rosenthal PJ, Donald Connolly J. Anti-plasmodial activity of some constituents of the root bark of Harungana madagascariensis LAM. (Hypericaceae). Chem Pharm Bull (Tokyo). 2007 Mar;55(3):464-7. doi: 10.1248/cpb.55.464. PMID: 17329893.
- Antia BS, Ita BN, Udo UE. Nutrient Composition and In Vitro Antioxidant Properties of Harungana madagascariensis Stembark Extracts. J Med Food. 2015 May;18(5):609-14. doi: 10.1089/jmf.2014.0084. Epub 2015 Mar 18. PMID: 25785542; PMCID: PMC4410548.
- Oluwadunmi O, Adebiyi OE, Abatan MO, Adedapo AA. Methanol and diethyl stem bark extracts of Harungana madagascariensis blunt acetaminophen-induced liver damage in rats through its antioxidant property. Savannah Veterinary Journal. Savannah Veterinary. 2019; 2:1–4.
- Happi GM, Tiani GLM, Gbetnkom BYM, Hussain H, Green IR, Ngadjui BT, Kouam SF. Phytochemistry and pharmacology of Harungana madagascariensis: mini-review. Phytochemistry Letters, 35(November). 2019; 103–112.
- Shorinwa OA, Monsi B. Toxicological implications of the fruit of Harungana madagascariensis on Wistar rats. Clin Phytosci. 2020; 6:2.
- Irvine FR. Woody plants of Ghana. Woody plants of Ghana. Oxford University Press, London. 1961; 143-144.
- Olagunju JA, Oladunni SO, Oladimeji MS. Status of phosphatase activities in the liver and kidney of rats treated with isosaline leaf and stem-bark extracts of Harungana madagascariensis (L). Cytobios. 2000;103(402):17-24. PMID: 11030222.
- Prajapati ND, Purohit SS, Sharma AK, Kumar T. A handbook of medicinal plants: A complete sourcebook. In A handbook of medicinal plants: a complete sourcebook. 2003; 554-554.
- Okoli AS, Okeke MI, Iroegbu CU, Ebo PU. Antibacterial activity of Harungana madagascariensis leaf extracts. Phytother Res. 2002 Mar;16(2):174-9. doi: 10.1002/ptr.991. PMID: 11933123.
- Iwalewa EO, Suleiman MM, Mdee KL, Eloff NJ. Antifungal and antibacterial activities of different extracts of Harungana madagascariensis stem bark, Pharmaceutical Biology. 2009; 47(9):878-885.
- Kengni F, Fodouop SP, Tala DS, Djimeli MN, Fokunang C, Gatsing D. Antityphoid properties and toxicity evaluation of Harungana madagascariensis Lam (Hypericaceae) aqueous leaf extract. J Ethnopharmacol. 2016 Feb 17;179:137-45. doi: 10.1016/j.jep.2015.12.037. Epub 2015 Dec 22. PMID: 26721224.
- Johnson-Ajinwo OR, Richardson A, Li WW. Palmatine from Unexplored Rutidea parviflora Showed Cytotoxicity and Induction of Apoptosis in Human Ovarian Cancer Cells. Toxins (Basel). 2019 Apr 25;11(4):237. doi: 10.3390/toxins11040237. PMID: 31027283; PMCID: PMC6521182.
- Burkill HM. The useful plants of West Tropical Africa. Vol. 1. Families AD (No. Ed. 2). Royal Botanic Gardens. 1985.
- Oberlies NH, Rogers LL, Martin JM, McLaughlin JL. Cytotoxic and insecticidal constituents of the unripe fruit of Persea americana. J Nat Prod. 1998 Jun 26;61(6):781-5. doi: 10.1021/np9800304. PMID: 9644064.
- Syahmi AR, Vijayarathna S, Sasidharan S, Latha LY, Kwan YP, Lau YL, Shin LN, Chen Y. Acute oral toxicity and brine shrimp lethality of Elaeis guineensis Jacq., (oil palm leaf) methanol extract. Molecules. 2010 Nov 10;15(11):8111-21. doi: 10.3390/molecules15118111. PMID: 21072022; PMCID: PMC6259233.
- Hisem D, Hrouzek P, Tomek P, Tomšíčková J, Zapomělová E, Skácelová K, Lukešová A, Kopecký J. Cyanobacterial cytotoxicity versus toxicity to brine shrimp Artemia salina. Toxicon. 2011 Jan;57(1):76-83. doi: 10.1016/j.toxicon.2010.10.002. Epub 2010 Oct 12. PMID: 20946912.
- Michael AS, Thompson CG, Abramovitz M. Artemia salina as a Test Organism for Bioassay. Science. 1956 Mar 16;123(3194):464. doi: 10.1126/science.123.3194.464. PMID: 17775415.
- Gajbhiye SN, Hirota R. Toxicity of heavy metals to brine shrimp Artemia. Journal of the Indian Fisheries Association. 1990; 20:43-50.
- Spielman A, Williams CM. Lethal effects of synthetic juvenile hormone on larvae of the yellow fever mosquito, Aedes aegypti. Science. 1966 Nov 25;154(3752):1043-4. doi: 10.1126/science.154.3752.1043. PMID: 5919758.
- McLaughlin JL, Rogers LL, Anderson JE. The use of biological assays to evaluate botanicals. Drug Information Journal. 1998; 32(2):513-524.
- Galsky AG, Wilsey JP. Crown Gall Tumor Disc Bioassay: A POSSIBLE AID IN THE DETECTION OF COMPOUNDS WITH ANTITUMOR ACTIVITY. Plant Physiol. 1980 Feb;65(2):184-5. doi: 10.1104/pp.65.2.184. PMID: 16661157; PMCID: PMC440294.
- Sarah QS, Anny FC, Mir M. Brine shrimp lethality assay. Bangladesh Journal of Pharmacology. 2017; 12(2):186-189.
- Levan A. The Effect of Colchicine on Root Mitosis in Allium. Hereditas. 1938; 24(4):471–486.
- Fiskesjö G. The Allium test as a standard in environmental monitoring. Hereditas. 1985;102(1):99-112. doi: 10.1111/j.1601-5223.1985.tb00471.x. PMID: 3988545.
- Rank J. The Method of Allium Anaphase-Telophase Chromosome Aberration Assay. Ekologija Vilnius. 2003 1:38-42.
- Bonciu E, Firbas P, Fontanetti CS, Wusheng J, Karaismailoğlu C, Liu D, Menicucci F, Pesnya DS, Popescu A, Romanovsky AV, Schiff S, Ślusarczyk J, De Souza CP, Srivastava A, Sutan A, Papini A. An evaluation for the standardization of the Allium cepa test as cytotoxicity and genotoxicity assay. Caryologia. 2018; 71(3):191-209.
- Bonea D, Bonciu E. Cytogenetic effects induced by the fungicide Royal Flo to maize (Zea mays L.), Caryologia. 2017; 70(3):195-199.
- Daphedar A, Taranath TC. Characterization and cytotoxic effect of biogenic silver nanoparticles on mitotic chromosomes of Drimia polyantha (Blatt. & McCann) Stearn. Toxicol Rep. 2018 Aug 31;5:910-918. doi: 10.1016/j.toxrep.2018.08.018. PMID: 30211013; PMCID: PMC6129697.
- Trease and Evans Pharmacognosy. 15th Ed, India: Elseiver. 2002; 191-393.
- Fiskesjo G. Allium test for screening chemicals; evaluation of cytological parameters. Plants for environmental studies. 1997; 11: 307-333.
- Grant WF. Chromosome aberration assays in Allium. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1982 Nov;99(3):273-91. doi: 10.1016/0165-1110(82)90046-x. PMID: 7177154.
- Mohanka PR, Kumari P, Kumar B. Cytotoxicity Evaluation of Aqueous Extracts of Medicinal Plants. 2019; 5(2): 35-49.
- Haydar TF, Ang E Jr, Rakic P. Mitotic spindle rotation and mode of cell division in the developing telencephalon. Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2890-5. doi: 10.1073/pnas.0437969100. Epub 2003 Feb 14. PMID: 12589023; PMCID: PMC151436.
- Mazia D. Mitosis and the physiology of cell division. In The cel,l. 1961; 77-412. Academic Press.
- Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001 Jan;2(1):21-32. doi: 10.1038/35048096. PMID: 11413462.
- Fiskesjö G. The Allium test--an alternative in environmental studies: the relative toxicity of metal ions. Mutat Res. 1988 Feb;197(2):243-60. doi: 10.1016/0027-5107(88)90096-6. PMID: 3340086.
- Fiskesjö G. The Allium test in wastewater monitoring. Environmental toxicology and water quality. 1993; 8(3): 291-298.
- Godwin A, Akinpelu B, Makinde A, Aderogba M, Oyedapo O. Identification of n-Hexane Fraction Constituents of Archidium ohioense (Schimp. ex Mull) Extract Using GC-MS Technique. British Journal of Pharmaceutical Research. 2015; 6(6): 366–375.
- Abubakar MN, Majinda RRT. GC-MS Analysis and Preliminary Antimicrobial Activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines (Basel). 2016 Jan 28;3(1):3. doi: 10.3390/medicines3010003. PMID: 28930113; PMCID: PMC5456228.
- Adegoke AS, Jerry OV, Ademola OG. GC-MS Analysis of Phytochemical Constituents in Methanol Extract of Wood Bark from Durio Zibethinus Murr. International Journal of Medicinal Plants and Natural Products. 2019; 5(3): 1-11.
- Ganesh M, Mohankumar M. Extraction and identification of bioactive components in Sida cordata (Burm.f.) using gas chromatography-mass spectrometry. J Food Sci Technol. 2017 Sep;54(10):3082-3091. doi: 10.1007/s13197-017-2744-z. Epub 2017 Jul 17. PMID: 28974793; PMCID: PMC5602971.
- Su Z, Wang P, Yuan W, Li S. Chemical constituents from Pterocarpus soyauxii. Nat Prod Commun. 2014 Oct;9(10):1483-6. PMID: 25522541.
- Rauter A, Ennis M, Hellwich K, Herold B, Horton D, Moss G, Schomburg I. Nomenclature of flavonoids (IUPAC Recommendations 2017). Pure and Applied Chemistry. 2018; 90(9): 1429-1486.
- Parveen Z, Nawaz S, Siddique S, Shahzad K. Composition and Antimicrobial Activity of the Essential Oil from Leaves of Curcuma longa L. Kasur Variety. Indian J Pharm Sci. 2013 Jan;75(1):117-22. doi: 10.4103/0250-474X.113544. PMID: 23901173; PMCID: PMC3719142.
- Pinto MEA, Araújo SG, Morais MI, Sá NP, Lima CM, Rosa CA, Siqueira EP, Johann S, Lima LARS. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. An Acad Bras Cienc. 2017 Jul-Sep;89(3):1671-1681. doi: 10.1590/0001-3765201720160908. Epub 2017 Aug 31. PMID: 28876392.
- Yu FR, Lian XZ, Guo HY, McGuire PM, Li RD, Wang R, Yu FH. Isolation and characterization of methyl esters and derivatives from Euphorbia kansui (Euphorbiaceae) and their inhibitory effects on the human SGC-7901 cells. J Pharm Pharm Sci. 2005 Sep 27;8(3):528-35. PMID: 16401398.
- Abdel KM, Ezdehar A, Shaza A. Parkinsonia aculeate Oil: GC-MS Analysis and Antimicrobial Activity. The Pharmaceutical and Chemical Journal. 2019; 6(5): 35-39.
- Rivas da Silva AC, Lopes PM, Barros de Azevedo MM, Costa DC, Alviano CS, Alviano DS. Biological activities of α-pinene and β-pinene enantiomers. Molecules. 2012 May 25;17(6):6305-16. doi: 10.3390/molecules17066305. PMID: 22634841; PMCID: PMC6268778.
- Mondello F, De Bernardis F, Girolamo A, Cassone A, Salvatore G. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis. 2006 Nov 3;6:158. doi: 10.1186/1471-2334-6-158. PMID: 17083732; PMCID: PMC1637110.
- Khaleel C, Tabanca N, Buchbauer G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chemistry. 2018; 16(1): 349-361.
- Nithya M, Ragavendran C, Natarajan D. Antibacterial and free radical scavenging activity of a medicinal plant Solanum xanthocarpum. International Journal of Food Properties. 2018; 21(1): 313-327.
- Sreedharan S, Gothe A, Aier K, Shivasharanappa K, Kumar KP, Patil SJ. Bioactive Molecules and Antimicrobial Studies of Indian Traditional Medicinal Plant Rhus semialata Seeds. Research Journal of Medicinal Plants Research Article. 2020; 1819-3455.
- Semwal P, Painuli S, Badoni H, Bacheti RK. Screening of phytoconstituents and antibacterial activity of leaves and bark of Quercus leucotrichophora A. Camus from Uttarakhand Himalaya, Clin Phytosci. 2018; 4: 30.